Search found 152 matches
- Sun Mar 14, 2021 10:57 pm
- Forum: Student Social/Study Group
- Topic: On Campus class vs. remote
- Replies: 21
- Views: 1458
Re: On Campus class vs. remote
I doubt that there will be the option to go fully remote. I know UCR is going for maximum in person classes next fall.
- Sun Mar 14, 2021 10:47 pm
- Forum: General Science Questions
- Topic: No Lavelle Chem 14C?
- Replies: 68
- Views: 4781
Re: No Lavelle Chem 14C?
Does anyone have an idea when they will take 14CL? I wanted to take it in the summer but I can't enroll yet.
- Sun Mar 14, 2021 10:45 pm
- Forum: General Science Questions
- Topic: Final thoughts
- Replies: 28
- Views: 4749
Re: Final thoughts
I thought the final was pretty simple. Not as many calculations as I expected.
- Sun Mar 07, 2021 11:30 pm
- Forum: General Rate Laws
- Topic: half life and rate law/reaction order
- Replies: 3
- Views: 236
Re: half life and rate law/reaction order
You have to do a qualitative analysis of the scenario in the problem. Since half life increases when the initial conc. decreases, we can infer that the reaction is second order since the 2nd order half life formula has initial conc. in the denominator.
- Sun Mar 07, 2021 11:25 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547143
Re: Saying Thank You to Dr. Lavelle
Once again, thank you Dr. Lavelle for another amazing quarter in general chemistry. I'm confident that taking 14A and 14B with you has thoroughly prepared me for future courses, exams, and studies in STEM.
- Sun Mar 07, 2021 11:19 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Sapling Week 9/10 Question 18
- Replies: 6
- Views: 668
Re: Sapling Week 9/10 Question 18
Intermediate species are those which are produced from one elementary step and consumed in the next step. They should not appear in the overall reaction since they cancel out. The same goes with a catalyst although I'm unfamiliar with the exact differences between the two.
- Sun Mar 07, 2021 11:15 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Knowing which formula to use
- Replies: 4
- Views: 371
Re: Knowing which formula to use
Understanding the conceptual reasoning behind each problem will be of great help. Its one thing to know how to plug in numbers to an equation and another thing to know when to apply it. Thermodynamics is perhaps the best example of this at work.
- Sun Mar 07, 2021 4:50 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Conc. Cell Solutions
- Replies: 4
- Views: 284
Conc. Cell Solutions
If the solution concentrations at the anode and cathode are equal for a concentration cell, does 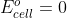 or
or 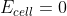 .
.
- Sun Feb 28, 2021 8:02 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing cell diagrams
- Replies: 3
- Views: 251
Re: Writing cell diagrams
We include H+ when the redox reaction occurs in acidic conditions. Similarly, we use OH- when the reaction in is basic conditions.
- Sun Feb 28, 2021 8:00 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: How Electricity is Quantified
- Replies: 3
- Views: 282
Re: How Electricity is Quantified
The electromotive force or (emf) is what causes a flow of electrons. It occurs when there is a electric potential difference between 2 points, in our case the anode and cathode. Emf is synonymous with voltage and it is measured in volts (joules/coloumb). Think of voltage/emf as the work/energy neede...
- Sun Feb 28, 2021 7:54 pm
- Forum: Balancing Redox Reactions
- Topic: Determining Phases
- Replies: 28
- Views: 1069
Re: Determining Phases
The anode and cathode will be solid state unless they are dissolved in the solution, then they are aqueous. OH- in basic conditions and H+ in acidic conditions will both be aqueous as well. Finally H20 will be liquid in galvanic cells.
- Sun Feb 28, 2021 7:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: anode vs. cathode
- Replies: 12
- Views: 734
Re: anode vs. cathode
The anode is shown with a negative charge and the anode dissolves into the solution by releasing cations through oxidation. The cathode grows in size due to the accumulation of metal on its surface from the reduction reaction.
- Fri Feb 26, 2021 10:38 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Diamond vs. Graphite
- Replies: 23
- Views: 1109
Diamond vs. Graphite
Lavelle mentioned in lectured today that the process from diamonds into graphite is thermodynamically favorable but doesn't occur due to a high energy barrier. Therefore is it still possible that diamonds can turn into graphite at standard conditions (room temp and 1 atm) over many many years?
- Mon Feb 22, 2021 3:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation @ equilibrium
- Replies: 6
- Views: 372
Re: Nernst Equation @ equilibrium
So E naught does not equal zero at equilibrium correct?
- Sun Feb 21, 2021 2:24 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Charging a cell [ENDORSED]
- Replies: 1
- Views: 204
Charging a cell [ENDORSED]
I believed Lavelle mentioned before that charging a cell is a non spontaneous process, which makes sense since my phone doesn't recharge itself. But mathematically, how is this proven?
- Sun Feb 21, 2021 2:20 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge Purpose
- Replies: 8
- Views: 568
Salt Bridge Purpose
I was a little confused after watching the lectures why the salt bridge is needed in a cell/battery. Also, how do the ions move from one side to another with the bridge?
- Thu Feb 18, 2021 10:39 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Delta S for Isothermal
- Replies: 8
- Views: 522
Re: Delta S for Isothermal
I believe it can be used for both since we approximate systems as being reversible.
- Wed Feb 17, 2021 11:11 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive properties
- Replies: 2
- Views: 207
Extensive properties
What are some thermodynamic properties that are considered extensive aside from entropy?
- Wed Feb 17, 2021 10:55 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Irreversible Expansion Entropy Change
- Replies: 2
- Views: 226
Re: Irreversible Expansion Entropy Change
Oh my gosh, I made the same mistake by using Cp. Thank you!
- Wed Feb 17, 2021 10:23 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Phase Change
- Replies: 3
- Views: 258
Re: Phase Change
I believe the person above is a little mistaken. The delta S of the SYSTEM is not equal to 0 but delta S TOTAL is in fact equal to zero since for a reversible reaction (which phases changes are since they use qrev) the delta S total is always equal to zero.
- Wed Feb 17, 2021 10:14 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Change in Enthalpy at Constant Volume
- Replies: 4
- Views: 253
Re: Change in Enthalpy at Constant Volume
Daniel Huynh 2J wrote:RdeltaT represents the work done on the system, n just represents the amount of moles that does that amount of work.
I thought when change in volume = 0, there is no work done. I'm a bit confused why this term is necessary then?
- Wed Feb 17, 2021 10:10 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q = n(ΔH) equation
- Replies: 2
- Views: 6090
Re: q = n(ΔH) equation
This equation can be used to calculate the heat required to perform a phase change of a particular substance. The number of moles of the substance multiplied by the enthalpy change for fusion or vaporization will give you how much heat is required to fully change that amount of a substance into a di...
- Wed Feb 17, 2021 10:05 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Irreversible Expansion Entropy Change
- Replies: 2
- Views: 226
Irreversible Expansion Entropy Change
For this problem, I approached it by calculating the change in entropy due to the change in volume and then added the change in entropy due to the increase in temperature. However, I didn't arrive at the correct answer of +11.2 J/K and I'm not sure why.
- Mon Feb 15, 2021 5:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Change in Enthalpy at Constant Volume
- Replies: 4
- Views: 253
Change in Enthalpy at Constant Volume
When I was reviewing the answers to some textbook problems I found this equation for the change in enthalpy at a constant volume: \Delta H=\Delta U + nR\Delta T Where does the second term in this equation come from? I understand that when the volume is constant, work=0 but I do not understand why th...
- Sun Feb 14, 2021 11:42 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Internal Energy, When Q is zero?
- Replies: 4
- Views: 356
Re: Internal Energy, When Q is zero?
I believe you are correct. A positive value for heat means there is an input of heat into the system from the surroundings. A negative value means the system released heat into the surroundings.
- Sun Feb 14, 2021 11:27 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Week 5/6 sapling 17
- Replies: 3
- Views: 520
Re: Week 5/6 sapling 17
So assuming that the standard entropy and free energy of the products and reactant is given in the table, all you need to do is follow this formula to calculate the change: (Sum of all standard entropies of the products) - (standard entropy of the reactant) The same applies with Gibbs free energy: (...
- Sat Feb 13, 2021 7:37 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Conceptual Explanation of ∆S = q/T
- Replies: 2
- Views: 226
Re: Conceptual Explanation of ∆S = q/T
When the temperature is higher, the molecules have more kinetic energy and therefore can occupy more micro-states. This is the reason why gases have higher entropy values. If the temperature is already high, adding more heat to the system will not lead to a dramatic increase the kinetic energy since...
- Sat Feb 13, 2021 7:30 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Sapling # 14
- Replies: 6
- Views: 381
Re: Sapling # 14
If you know both the change in enthalpy of vaporization and the change in entropy of vaporization you can use the equation \Delta S=\frac{q_{rev}}{T} Since enthalpy is just the heat at constant pressure you can replace q with the value of the change in enthalpy and solve for T which will be the temp...
- Thu Feb 11, 2021 12:37 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 2 Coverage
- Replies: 6
- Views: 403
Re: Midterm 2 Coverage
The rest of thermochemistry and up until the end of the thermodynamics outline I believe but Lavelle will let us know for sure.
- Thu Feb 11, 2021 12:20 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Textbook Problem 4H.9
- Replies: 3
- Views: 128
Re: Textbook Problem 4H.9
In class, we discussed that the change in entropy is lower when adding heat to a substance already at a high temperature. I think you may be confusing this concept with changing entropy of substances at low vs high entropy. Changes in temperature mostly have to do with changing the kinetic energy o...
- Wed Feb 10, 2021 6:42 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Textbook Problem 4H.9
- Replies: 3
- Views: 128
Textbook Problem 4H.9
I was confused on the solution to this problem which listed the increasing change in entropies as B<C<A. I thought the order would be C<B<A since molecules in C are already vibrationally active thus start out with a larger entropy value. Wouldn't this mean that their change in entropy when raised to...
- Wed Feb 10, 2021 1:26 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Today's lecture
- Replies: 5
- Views: 279
Re: Today's lecture
I believe that is correct. That's how I understood it.
- Wed Feb 10, 2021 12:28 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy of Surroundings
- Replies: 4
- Views: 274
Entropy of Surroundings
In lecture today Lavelle gave an equation of how to calculate entropy of surroundings: \Delta S {surr}=-\frac{\Delta H_{sys}}{T} Is the temperature T in this equation the temperature of the system or of the surroundings? I was confused because when he was showing an example of thermal equilibrium it...
- Sun Feb 07, 2021 11:21 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: using equations
- Replies: 11
- Views: 629
Re: using equations
You have to use context clues that are given in the question. Often in Thermodynamics problems, you are supposed to draw your own conclusions based on "hidden" information in the problem. For example, when pressure is constant, q becomes the definition of enthalpy so you could replace it w...
- Sun Feb 07, 2021 11:17 pm
- Forum: Ideal Gases
- Topic: Chem BL
- Replies: 107
- Views: 8071
Re: Chem BL
I was planning on taking Chem BL and CL together over the summer. Is this a bad idea to take two lab courses at once?
- Sat Feb 06, 2021 10:59 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Work on a system
- Replies: 27
- Views: 1125
Work on a system
What is an example where work is done on the system, resulting in a positive value for work?
- Sat Feb 06, 2021 10:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: specific vs molar heat capacity
- Replies: 16
- Views: 936
Re: specific vs molar heat capacity
Molar heat capacity can be thought of as a form of specific heat capacity. Specific heat capacity is the energy required to raise the temperature by 1 degree for a GRAM of that substance. Molar heat capacity is the energy required to raise the temperature by 1 degree for a MOLE of that substance.
- Sat Feb 06, 2021 10:52 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Standard Entropy
- Replies: 2
- Views: 101
Standard Entropy
I've been seeing a lot of examples which mention the standard entropy of vaporization for a substance. What does this term exactly mean? Is this just the entropy when the liquid is vaporizing?
- Sat Feb 06, 2021 10:48 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy and Volume Relationship
- Replies: 1
- Views: 95
Re: Entropy and Volume Relationship
I believe this has to do with the space the gas molecules can now occupy which is similar to the 2 chamber vial example Lavelle showed us when he defined degeneracy. In Lavelle's example, the gas molecules could either be split between the two chambers or in one of the chambers. Doubling the volume ...
- Wed Feb 03, 2021 12:54 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Microstates vs. Possible positions
- Replies: 3
- Views: 115
Re: Microstates vs. Possible positions
I should clarify. The degeneracy is after you evaluate the expression and is the number of possible arrangements. Like in todays lecture, the 2 states of left and right with 2 particles has 4 possible ways to arrange it.
- Wed Feb 03, 2021 12:50 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Microstates vs. Possible positions
- Replies: 3
- Views: 115
Re: Microstates vs. Possible positions
I believe the terms are synonyms for one another. You can say arrangements, microstates, etc. It just means the number of possible outcomes/final states. For example left or right or on or off.
- Wed Feb 03, 2021 12:45 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Residual Entropy=0
- Replies: 1
- Views: 275
Residual Entropy=0
In lecture today, Dr. Lavelle showed an example of an O2 molecule having a residual entropy of 0 since it only has one state. Does this mean all diatomic molecules like F2 and Cl2 will also have a residual entropy of 0? What about BF3? or CH4?
- Mon Feb 01, 2021 1:10 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Change in Internal Energy Formula
- Replies: 3
- Views: 231
Change in Internal Energy Formula
In the lecture today Dr. Lavelle substituted work as the function 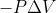 . Does this mean we can substitute in the formula for a reversible reaction under conditions of constant pressure? The one with the integral.
. Does this mean we can substitute in the formula for a reversible reaction under conditions of constant pressure? The one with the integral.
- Sun Jan 31, 2021 10:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Higher Enthalpy
- Replies: 6
- Views: 216
Re: Higher Enthalpy
Yes if the change in enthalpy is negative, it is an endothermic reaction and heating the system will produce less product. :)
- Sun Jan 31, 2021 10:57 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: divide heat capacity by amount represent
- Replies: 4
- Views: 194
Re: divide heat capacity by amount represent
What context are you asking your question for? Like what problem?
- Sun Jan 31, 2021 10:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Closed vs Isolated Systems
- Replies: 4
- Views: 236
Re: Closed vs Isolated Systems
Think of a closed water bottle for a closed system and a closed thermos/canteen for an isolated system.
- Fri Jan 29, 2021 9:20 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: R Constant
- Replies: 91
- Views: 6208
Re: R Constant
Yes, look at the units the problem gives you. Most of the time though it will be atm. Your equation sheet will have all of them though.
- Fri Jan 29, 2021 9:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka and Kb
- Replies: 9
- Views: 435
Re: Ka and Kb
You need to know whether the salt has the anion of a conjugate base from a weak acid or an anion of a conjugate acid from a weak base. Remember that halogens do not affect the pH nor do alkali earth metal cations.
- Fri Jan 29, 2021 9:14 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Neutralization
- Replies: 25
- Views: 1066
Re: Neutralization
Neutralization is where an acid and a base combine to form a salt in water. The pH will be neutral in such cases. Combining HCl and NaOH will result in the formation of NaCl and water, neither of which will affect the pH of the solution.
- Fri Jan 29, 2021 9:04 pm
- Forum: Calculating Work of Expansion
- Topic: Sapling #15
- Replies: 3
- Views: 158
Sapling #15
When I calculated the volume of N2 gas in the airbag, I use the 1 atm as my pressure in the ideal gas equation. So in the next step where I use the equation 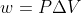
, what value do I use for my pressure? I can't seem to get the right answer.
, what value do I use for my pressure? I can't seem to get the right answer.
- Thu Jan 28, 2021 1:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka and Kb
- Replies: 2
- Views: 230
Ka and Kb
In an acid-base reaction such as NH4+ giving off a proton to turn into NH3. Would 1/Ka of this reaction be equal to the Kb for the reverse reaction where the conjugate base NH3 accepts a proton?
- Sun Jan 24, 2021 6:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Polyprotic Acids
- Replies: 5
- Views: 290
Re: Polyprotic Acids
From what I understand, Sulfuric acid can be considered completely disassociated/ionized in its first K value as it is on the list of strong acids. As to how close K1 and K2 need to be, I am not sure.
- Fri Jan 22, 2021 4:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Preferred way to calculate enthalpy
- Replies: 6
- Views: 402
Re: Preferred way to calculate enthalpy
To use Hess's Law, you need to be given equations (and their corresponding enthalpies) that can be related to your equation of interest. To use standard enthalpy, you need to have a table of the enthalpies of common compounds such as CO or H20. As for which one is more accurate I am not quite sure.
- Fri Jan 22, 2021 2:56 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Sapling Week 2 #5
- Replies: 3
- Views: 332
Re: Sapling Week 2 #5
Try setting up your Kb expression now. Since you know your OH- concentration, you know that your NH4+ concentration must be the same which will allow you to solve for the NH3 concentration. Once you find NH3, that is your EQUILIBRIUM concentration so you still need to find your initial in order to c...
- Fri Jan 22, 2021 2:34 pm
- Forum: General Science Questions
- Topic: Lecture Question
- Replies: 4
- Views: 223
Re: Lecture Question
The enthalpies for bonds that are not diatomic are averaged across all the other molecules they are found in. Therefore, using bond enthalpies is the least accurate method of the three ways Dr. Lavelle showed us.
- Fri Jan 22, 2021 2:32 pm
- Forum: General Science Questions
- Topic: Lecture Question
- Replies: 4
- Views: 223
Re: Lecture Question
He gets them from a pre made table which lists the enthalpies of known bonds. He changes the sign for the enthalpies in the products since forming a bond releases energy and is exothermic.
- Fri Jan 22, 2021 2:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Knowing which bonds are broken and formed
- Replies: 4
- Views: 574
Re: Knowing which bonds are broken and formed
Yes drawing out the lewis structures is necessary in more advanced cases. It helps visualize bounds that were broken and reformed such as the double bond of carbon in today's example in class.
- Fri Jan 22, 2021 2:20 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Graphite
- Replies: 3
- Views: 201
Graphite
Why is carbon's most stable state graphite? Is the graphite In my pencil pure carbon lol?
- Sun Jan 17, 2021 8:46 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 18555
Re: Exercising Our Minds and Bodies
I go for walks with my girlfriend and play with my cat Lucy :)
- Sun Jan 17, 2021 8:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling HW Week 1 #5
- Replies: 8
- Views: 2271
Re: Sapling HW Week 1 #5
You have to combine the equations it gives you to make the correct equation in the question. You can inverse their equations and even multiply them by stoichiometric constants .
- Sun Jan 17, 2021 8:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acid and Bases
- Replies: 3
- Views: 207
Re: Acid and Bases
If you are not given the formula or if it is already balanced then I think so.
- Sun Jan 17, 2021 8:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating pH given the concentration
- Replies: 6
- Views: 248
Re: Calculating pH given the concentration
The concentration of H30+ or OH- should never surpass 1 or go lower than 1x10^-14. The autoprotolysis of water explains that at 25 degrees C, the product of H30 and OH is always 10^-14 (hence the pH scale). You shouldn't have a H30 concentration above 1.
- Sun Jan 17, 2021 8:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Picking answer from quadratic solutions
- Replies: 12
- Views: 843
Re: Picking answer from quadratic solutions
Sometimes an x value will be too large when subtracting x from the reactants and would give you negative reactants which isn't possible.
- Sun Jan 17, 2021 5:09 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature
- Replies: 45
- Views: 1431
Re: Temperature
Based on a given enthalpy (usually seen as delta h)
- Fri Jan 15, 2021 11:47 am
- Forum: Student Social/Study Group
- Topic: Midterms During Lecture
- Replies: 44
- Views: 1993
Re: Midterms During Lecture
Lindsey_Su_3A wrote:Does anyone know how long the midterms will be?
90 minute midterms
- Fri Jan 15, 2021 11:44 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Week 1 Sapling #9
- Replies: 5
- Views: 325
Re: Week 1 Sapling #9
Begin by finding the Kc (equilibrium constant) based on the given concentrations. After, make an ice table with the initial concentration of NO2 being 0.8 M while O2 and N2 remain at 0.2 M. Since more product was added to the system, there will be a shift toward the reactants. Therefore you can crea...
- Thu Jan 14, 2021 9:20 am
- Forum: Student Social/Study Group
- Topic: New Joke Chain
- Replies: 46
- Views: 3083
Re: New Joke Chain
TJ Lai 2H wrote:Nick P 3D wrote:H2O is water and H2O2 is hydrogen peroxide.
What is H2O4?
Drinking
Can someone explain this joke? I don't get it :(
Same with this from lecture yesterday:
Screenshot 2021-01-14 103034.png
Instead of saying “what is H204?”
Say it as “what is H20 for”
- Wed Jan 13, 2021 6:14 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589211
Re: Post All Chemistry Jokes Here
What do Switzerland and H20 have in common?
They always remain neutral!
They always remain neutral!
- Wed Jan 13, 2021 6:11 pm
- Forum: Student Social/Study Group
- Topic: New Joke Chain
- Replies: 46
- Views: 3083
Re: New Joke Chain
What do Switzerland and H20 have in common?
They are always neutral!
They are always neutral!
- Wed Jan 13, 2021 6:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka equation for diprotic acids
- Replies: 2
- Views: 616
Ka equation for diprotic acids
My understanding is that monoprotic acids forms only one mole of H30+ per molecule of the acid. Therefore in the Ka equation, there should be no exponents on the concentrations of H30+. Would this mean that in a diprotic acid such as H2SO4, would there be a 2 on the exponent of the H30+ concentratio...
- Fri Jan 08, 2021 11:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increasing Pressure by Adding an Inert Gas
- Replies: 6
- Views: 871
Re: Increasing Pressure by Adding an Inert Gas
Adding an inert gas does not affect the concentration of the reaction because concentration is determined by the equation moles/volume. Adding the inert gas into the reaction chamber does not affect the moles of reactants nor the volume of the chamber since the volume will be fixed. All it does is i...
- Thu Jan 07, 2021 4:39 pm
- Forum: Ideal Gases
- Topic: Reaction Quotient(Q) vs. Equilibrium Constant(K)
- Replies: 9
- Views: 1186
Re: Reaction Quotient(Q) vs. Equilibrium Constant(K)
One important thing to note is that many will refer to an equilibrium as shifted or leaning to the right or left. This does not mean the reaction is not at equilibrium, this means the forward reaction either favors products or reactants more. A Q value on the other hand can physically lay right or l...
- Thu Jan 07, 2021 4:35 pm
- Forum: Ideal Gases
- Topic: Kc vs Kp
- Replies: 109
- Views: 4791
Re: Kc vs Kp
It depends on what the question gives you and what it is asking for. If it gives you the concentration of a reactant or product, use Kc. If it gives you a pressure, use Kp.
- Thu Jan 07, 2021 4:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling #5
- Replies: 4
- Views: 186
Sapling #5
I am having trouble understanding where to begin on this question. Since no concentrations are given for any of the products or reactants, I know that the solution will have to involve using the other chemical equilibriums but I am unsure how to relate them. Any advice? Screen Shot 2021-01-07 at 4.3...
- Mon Jan 04, 2021 1:54 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Learning Issues
- Replies: 9
- Views: 200
Re: Sapling Learning Issues
Sorry, I never expected it to be a problem with the browser given that I used Safari all last quarter and ran into no issues. My apologizes for the inconvenience.
- Mon Jan 04, 2021 1:35 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Learning Issues
- Replies: 9
- Views: 200
Re: Sapling Learning Issues
Strange because I always used Safari last quarter and had already disabled my pop up blockers and it always worked but now it just refuses.
- Mon Jan 04, 2021 1:32 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Learning Issues
- Replies: 9
- Views: 200
Re: Sapling Learning Issues
Jazlyn Romero 1I wrote:This same issue happened to me when I tried accessing sapling through safari so I tried it on google chrome and it worked. Maybe try on google chrome or another browser?
Yes thank you that worked! For some reason Safari doesn't like Sapling lol
- Mon Jan 04, 2021 1:28 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Learning Issues
- Replies: 9
- Views: 200
Re: Sapling Learning Issues
Chem_Mod wrote:I contacted Sapling over the weekend and they said today (Monday) it should be fixed. Try again and it should work.
Im still receiving the same error that reads: "You must sign up for this through your instructor's course on your University's learning management system."
- Mon Jan 04, 2021 1:22 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Learning Issues
- Replies: 9
- Views: 200
Sapling Learning Issues
I have not been able to access the Sapling Learning homework for Chem 14B even though I bought a 24 month access last quarter for Chem 14A. I contacted the sapling support and they said it must be a issue on Lavelle's or UCLA's part. Has anyone also been getting an error when trying to enroll with y...
- Tue Dec 15, 2020 12:21 pm
- Forum: Lewis Acids & Bases
- Topic: Classifying Other
- Replies: 4
- Views: 227
Re: Classifying Other
Good to remember the short list of strong acids and bases
- Tue Dec 15, 2020 12:19 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547143
Re: Saying Thank You to Dr. Lavelle
Thank you Dr. Lavelle for making this course very well adapted for online learning! The review sessions and Chemistry community were of great help! I look forward to taking Chem 14B.
- Sat Dec 12, 2020 6:43 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Rules of Electron Config
- Replies: 6
- Views: 357
Re: Rules of Electron Config
Thank you! I had the impression it was Hund's rule but I never saw this type of violation where an electron was in the opposite spin state.
- Sat Dec 12, 2020 6:31 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Rules of Electron Config
- Replies: 6
- Views: 357
Rules of Electron Config
Which rule does this configuration violate? Is it Hund''s rule?
- Fri Dec 11, 2020 12:49 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook 6B.1
- Replies: 8
- Views: 380
Re: Textbook 6B.1
Take the -log of 12% to get 0.92
-log(0.12)
-log(0.12)
- Fri Dec 11, 2020 12:14 am
- Forum: Polyprotic Acids & Bases
- Topic: Which one is more acidic: H3PO3 or H3PO4
- Replies: 17
- Views: 1263
Re: Which one is more acidic: H3PO3 or H3PO4
I believe the stronger acid is H3PO4 since the resulting anion is more stable due to the xtra oxygen which increases electronegativity. PO4-3 also has resonance structures which delocalize its electrons to disperse the negative charge across the molecule.
- Fri Dec 11, 2020 12:06 am
- Forum: Polyprotic Acids & Bases
- Topic: Calculating Ka
- Replies: 9
- Views: 1124
Re: Calculating Ka
In this example, you would divide the product of the concentrations of the products over the concentration of the solvent: AH <—> A- (aq) + H+ (aq) leads to Ka =( [A-][H+] )/[AH] Concentrations, if they aren't given, would be calculated through our molarity formula M = n/V, where n=moles and v= vol...
- Fri Dec 11, 2020 12:03 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook 6B.1
- Replies: 8
- Views: 380
Re: Textbook 6B.1
Is that all the information the question gives?
- Fri Dec 11, 2020 12:02 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strong Acid Question
- Replies: 2
- Views: 208
Re: Strong Acid Question
When comparing two weak acids, you can determine their relative strength to one another by examining the hydrogen bond. If the bond is longer, it is also weaker and thus the hydrogen can easily dissociate (stronger acid). If the bond lengths are the same, you want to look at the stability of the res...
- Wed Dec 09, 2020 7:48 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sapling Week 9 #3
- Replies: 1
- Views: 97
Re: Sapling Week 9 #3
It is technically possible but it is very uncommon for these shapes to occur in coordination complexes. I was also confused at first since the see saw shape also has 4 bound atoms. Square planar and tetrahedral are just more common.
- Wed Dec 09, 2020 7:46 am
- Forum: Bronsted Acids & Bases
- Topic: Amphoteric compounds
- Replies: 8
- Views: 594
Re: Amphoteric compounds
Often conjugate bases can be made out of atoms that are metalloids such as Si, P, As, etc. HPO4- is one example of an amphoteric compound.
- Wed Dec 09, 2020 7:42 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Sapling Q #1
- Replies: 7
- Views: 339
Re: Sapling Q #1
Yeah the things we have to remember in this course are the name of ligands - charge and conformation. - (monodentate, bidentate etc.) and the strong acids/bases.
- Tue Dec 08, 2020 9:23 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angle
- Replies: 3
- Views: 217
Re: bond angel
I'm not quite sure. I know the most stable structure for molecules spread their electron densities as far away as possible, so the bond angle is always the largest possible angle. I know bond strengths are determined on their bond order and their length. They can also be weakened by atoms with large...
- Mon Dec 07, 2020 11:56 pm
- Forum: Bronsted Acids & Bases
- Topic: electronegativity and acidity
- Replies: 3
- Views: 247
Re: electronegativity and acidity
The order when examining the strength of an acid goes:
1.) Bond length of hydrogen
2.) Stability of resulting anion
a. Has an atom with a large electronegativity which pulls and disperses the negative charge
b. Has a resonance structure which delocalizes the electrons
1.) Bond length of hydrogen
2.) Stability of resulting anion
a. Has an atom with a large electronegativity which pulls and disperses the negative charge
b. Has a resonance structure which delocalizes the electrons
- Mon Dec 07, 2020 11:50 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Ka
- Replies: 1
- Views: 130
Ka
Why do we have to take the pKa value for some acids when we can take the pH for others? Do we only use the Ka value for weak acids? I know that a large pKa value means a stronger acid and a smaller pKa value means a stronger acid as well.
- Sun Dec 06, 2020 11:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle
- Replies: 4
- Views: 147
Re: Bond Angle
I like to make quizlet flashcards to help me remember :)
- Sun Dec 06, 2020 11:17 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: pH vs pOH
- Replies: 9
- Views: 417
pH vs pOH
Is there a pOH scale that would also go from 0 to 14 like pH?
- Sat Dec 05, 2020 11:00 pm
- Forum: Lewis Acids & Bases
- Topic: KA and pKA
- Replies: 19
- Views: 964
Re: KA and pKA
Also remember that the product between the concentration of H30+ molecules and the concentration of OH- molecules will always be 1*10^-14. Some questions may ask you to find the H3O+ conc. by giving you the OH- conc. Just useful to know (:
- Fri Dec 04, 2020 12:53 am
- Forum: Dipole Moments
- Topic: CH2Cl2 vs C6H4Cl2
- Replies: 3
- Views: 489
Re: CH2Cl2 vs C6H4Cl2
CH2Cl2 is a polar molecule because of its tetrahedral shape. No mater where you place the chlorine atoms, there will be a net dipole moment. C6H4Cl2 on the other hand is a cyclic structure meaning it forms a ring. In this structure, the chlorines are position are opposing ends which would cancel the...
- Fri Dec 04, 2020 12:42 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sapling Week 9 #5
- Replies: 3
- Views: 228
Re: Sapling Week 9 #5
First thing to remember is that the coordination number is the number of bonds or attachment points to the metal cation (in this case Cobalt) within the coordination sphere. The coordination sphere is indicated by the brackets so Bromine would not be included when finding the coordination number. No...
- Fri Dec 04, 2020 12:32 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: List of Ligands
- Replies: 5
- Views: 186
Re: List of Ligands
Yes you should definitely be ready to name the ligand if you are given its formula or abbreviation such as (en) or (edta). Also make sure you know the oxidation of the ligand so you can calculate the oxidative state of the metal cation. If you can't remember this, try quickly drawing the ligand to f...

