Search found 155 matches
- Wed Mar 10, 2021 11:54 pm
- Forum: General Rate Laws
- Topic: Liquid Water in Rate Laws
- Replies: 4
- Views: 278
Liquid Water in Rate Laws
Hi, I was wondering if we include liquid water in rate laws? Question 7.9 from the textbook seems to include water in the rate law, and I was confused by this. Thank you in advance!
- Mon Mar 08, 2021 2:46 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Microscopic Reversibility
- Replies: 1
- Views: 148
Microscopic Reversibility
How does the analysis of the equilibrium constant and the forward and reverse rates of a reaction change if we cannot assume microscopic reversibility?
- Mon Mar 08, 2021 2:43 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Theory [ENDORSED]
- Replies: 1
- Views: 397
Arrhenius Theory [ENDORSED]
The Arrhenius Theory was mentioned in lecture today to explain how reactions that follow it have linear plots with slope M=-Ea/R, but what is the Arrhenius Theory specifically?
- Mon Mar 08, 2021 2:40 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Low versus High Activation Energy
- Replies: 3
- Views: 227
Low versus High Activation Energy
In lecture today (03/08), a graph was shown for the Arrhenius equation in which a higher activation energy had a steeper negative slope than a reaction with a low activation energy. Could some explain why there is a difference in these graphs?
- Mon Mar 08, 2021 2:37 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Frequency Factor
- Replies: 3
- Views: 214
Frequency Factor
When considering the frequency factor in the Arrhenius equation, why is it important to the rate constant that collisions are in the correct orientation?
- Mon Mar 08, 2021 2:33 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation energy
- Replies: 6
- Views: 430
Re: Activation energy
If you use the R constant 8.314 J/mol K, then Ea would be in J/mol. Make sure to be match the units in the equation.
- Sun Mar 07, 2021 10:18 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Appendix 2B
- Replies: 3
- Views: 340
Re: Appendix 2B
Hi, for textbook problems, there are some problems that do want you to use Appendix 2B even though it might not be directly stated.
- Mon Mar 01, 2021 1:45 pm
- Forum: General Rate Laws
- Topic: Number of Reactants
- Replies: 26
- Views: 1111
Number of Reactants
Is it possible to have more than three reactants when determining a general rate law? In lecture today, it was said that n, m, and l are usually used as the exponents for reactants in a general rate law, so does that mean that we wouldn't see more than 3 reactants?
- Mon Mar 01, 2021 1:43 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Determining Time with Phases
- Replies: 4
- Views: 306
Determining Time with Phases
In lecture today, it was mentioned that in a reaction where N2 gas is a product, this means that t is close to t=0, but how do we know this? Why does a gas leaving a solution mean that the reverse reaction has a less chance of happening?
- Mon Mar 01, 2021 1:41 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Value of k throughout Experiments
- Replies: 5
- Views: 296
Value of k throughout Experiments
Is the value of k only specific to a certain moment in point of the reaction at which you are plugging in a certain experiment's values for concentrations? Or is k constant for a reaction in all experiments of that reaction?
- Mon Mar 01, 2021 1:39 pm
- Forum: Zero Order Reactions
- Topic: Occurrence of Zero Order Reactions
- Replies: 13
- Views: 775
Occurrence of Zero Order Reactions
When do zero order reactions occur mathematically? Is that saying that the exponents that are associated with concentrations are all 0 and thus the concentrations of reactants do not affect the rate law? Or what is the meaning of a zero order reaction?
- Mon Mar 01, 2021 1:37 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Reverse Reactions
- Replies: 7
- Views: 398
Reverse Reactions
In lecture today, it was mentioned that we will only be looking at the forward reaction, but why was it also mentioned that we need the rates of the forward reaction and the reverse reaction? Which one is the one given for initial rates when looking at concentration tables?
- Sun Feb 28, 2021 5:06 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Metalloids as Conductors
- Replies: 1
- Views: 155
Metalloids as Conductors
Hi, I know that nonmetals are not conductors of electricity, but when considering cell diagrams, are metalloids considered conductors of electricity? Or is it just metals or just specifically d-block metals? Thank you in advance!
- Fri Feb 26, 2021 12:34 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook 6M.1 error [ENDORSED]
- Replies: 2
- Views: 178
Re: Textbook 6M.1 error [ENDORSED]
I was also curious about this question! If there isn't an error, I'm wondering if it has to do with how Enaught of the cell is a negative value, which would indicate a non spontaneous process and thus favors the reverse reaction, meaning the cathode and anode or switch? I'm not sure if this makes se...
- Wed Feb 24, 2021 12:18 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Non-Spontaneous Reactions
- Replies: 3
- Views: 250
Non-Spontaneous Reactions
In today's lecture (02/24) at around 29:07, Dr. Lavelle says that there is a positive E naught value, which is why its a nonspontaneous reaction, but I thought positive E naught values were associated with spontaneous processes? Could someone explain this?
- Mon Feb 22, 2021 11:40 pm
- Forum: Balancing Redox Reactions
- Topic: W7/8 Sapling #18
- Replies: 3
- Views: 274
Re: W7/8 Sapling #18
Since the coefficient of 2 in front of the product in the equation is applied to the entire product, that means that you have 4 moles of Fe, 6 moles of O, 12 moles of H, and 6 moles of O, and so to balance this out, the H2O in your reactants should have a coefficient of 6 instead of a 3.
- Mon Feb 22, 2021 12:44 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Are More Reactants or Products are Favorable?
- Replies: 3
- Views: 257
Are More Reactants or Products are Favorable?
In lecture today, it was mentioned that having more products than reactants is thermodynamically favorable, as we have learned, but why is it that we should start with more reactants than products when setting up a voltage? What is the outcome if you start out with more products than reactants?
- Mon Feb 22, 2021 12:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Effect of Concentration on Cell Potential
- Replies: 2
- Views: 181
Effect of Concentration on Cell Potential
I was wondering how, in lecture today, we can tell that when the concentration of Mn2+ is increased, the cell potential is greater than the standard cell potential. Is this apparent from the chemical equation? I'm just not sure how to observe this.
- Mon Feb 22, 2021 12:35 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Voltage
- Replies: 4
- Views: 250
Re: Voltage
Yes, you are correct in that voltage is the maximum cell potential because it is a measure of the maximum cell potential in a battery.
- Mon Feb 22, 2021 12:32 pm
- Forum: Balancing Redox Reactions
- Topic: Adding H+, OH-, and H2O in Redox Reactions
- Replies: 7
- Views: 5355
Adding H+, OH-, and H2O in Redox Reactions
On many questions that I have seen with balancing redox reactions, I have seen H+, OH-, and H2O being added, but I'm not sure where these came from or how to add them to reactions. When do you know to add one of these species?
- Mon Feb 22, 2021 12:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Is a non spontaneous reaction possible?
- Replies: 4
- Views: 296
Re: Is a non spontaneous reaction possible?
Dr. Lavelle mentioned in lecture that you cannot have a negative cell potential, as you would never find a battery with a negative cell potential, so you may be right in your assumption that it would not be possible to have a non spontaneous reaction. At the very least, non spontaneous reactions are...
- Wed Feb 17, 2021 8:44 am
- Forum: Calculating Work of Expansion
- Topic: When to use each work equatin?
- Replies: 2
- Views: 171
Re: When to use each work equatin?
Yes, w = -(Pex)(deltaV) is used for irreversible reactions and w = -nRTln(v2/v1) is used for isothermal, reversible reactions. Hope this helps!
- Tue Feb 16, 2021 2:22 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: How to solve for q at constant volume?
- Replies: 3
- Views: 355
Re: How to solve for q at constant volume?
In the textbook, there was a question asking us to solve for q at constant volume of an ideal gas. To do this, you just use q(v) = nC(v)ΔT, using C(v)=(3/2)R, which is the heat capacity of an ideal gas at constant volume. C(v) is given on the equation sheet. To solve for q at constant pressure, do ...
- Tue Feb 16, 2021 11:35 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Change with Pressure
- Replies: 3
- Views: 291
Re: Entropy Change with Pressure
You can use the equation 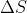 = n*R*ln(P1/P2).
= n*R*ln(P1/P2).
- Tue Feb 16, 2021 11:24 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: textbook problem 4c. 15
- Replies: 2
- Views: 159
Re: textbook problem 4c. 15
You would be looking for steeper slopes when considering that the solid and the gas take less time to heat up, so their slope has to be steeper so that the heating curve would accurately represent those forms getting to the melted or vaporized form, respectively.
- Tue Feb 16, 2021 11:13 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Textbook 4I.9
- Replies: 2
- Views: 161
Re: Textbook 4I.9
It is important to remember that in reversible processes, delta S total is 0 because this process is at equilibrium. However, in irreversible processes, delta S surroundings is 0. So, for a reversible process, the equation of deltaS total = delta S system + delta S surroundings would be 0 = delta S ...
- Tue Feb 16, 2021 11:09 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Heat at a constant pressure and enthalpy
- Replies: 4
- Views: 260
Re: Heat at a constant pressure and enthalpy
Heat can be thought of when looking at certain conditions. The value of q is more of a general value that accounts for a changing pressure, but enthalpy is a form of that heat that is at constant pressure. Hope this helps!
- Tue Feb 16, 2021 11:07 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: textbook 4C.3
- Replies: 2
- Views: 224
Re: textbook 4C.3
You use those equations that include values for Cp and Cv because these are the specific heat capacities that account for the pressure or volume being held constant, respectively. The values for Cp and Cv can be found on the constants sheet, and you can proceed with the rest of the values given in t...
- Fri Feb 12, 2021 11:58 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs and Equilbrium constant
- Replies: 3
- Views: 164
Re: Gibbs and Equilbrium constant
You can relate Gibbs free energy and the equilibrium constant through the equation \Delta Gnaught = -RTlnK which is derived from the equation deltaG = delta G naught + RTlnQ, where Q is the reaction quotient and K is the equilbirum constant. The first equation is true when there is equilibrium, as d...
- Fri Feb 12, 2021 11:55 am
- Forum: Calculating Work of Expansion
- Topic: Irreversible isothermal expansion?
- Replies: 1
- Views: 165
Re: Irreversible isothermal expansion?
Yes, there should be some irreversible processes that could be isothermal. I'd also be interested in knowing what some examples are of this, though.
- Mon Feb 08, 2021 12:16 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Assuming 298 K
- Replies: 7
- Views: 306
Re: Assuming 298 K
Standard temperature and pressure is 25 degrees C or 298 K and 1 atm, respectively, which should be able to be assumed if you are not given any other conditions instead.
- Mon Feb 08, 2021 12:07 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Entropy in an Isolated System
- Replies: 4
- Views: 238
Entropy in an Isolated System
Can entropy change as a result of reactions in an isolated system? Since heat and energy cannot be exchanged between an isolated system and its surroundings, what happens to the entropy of the surroundings?
- Mon Feb 08, 2021 12:05 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Using T1 and T2 for an isothermal process
- Replies: 4
- Views: 321
Using T1 and T2 for an isothermal process
I am still confused about how we can take values for T1 and T2 to calculate an isothermal process as shown in today's lecture (Lecture #14). Aren't isothermal processes held at a constant temperature? Is there a difference between T1 and T2?
- Mon Feb 08, 2021 12:03 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Which R to use
- Replies: 42
- Views: 3019
Re: Which R to use
Adding onto what has already been said about using 8.314 J/mol*K, make sure to always write out the units of your values because even if you do not remember which R value to use, the units can really help you identify which R value will work out.
- Mon Feb 08, 2021 12:02 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Progress of Rxn Diagram
- Replies: 4
- Views: 266
Re: Progress of Rxn Diagram
I think your placement is correct, but the question wants the spontaneity to be placed in the above boxes rather than the bottom. However, your understanding of spontaneity and the relationship between Q and K is correct.
- Mon Feb 08, 2021 11:59 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible reaction effeciency
- Replies: 4
- Views: 224
Re: Reversible reaction effeciency
Reversible reactions do more work, actually, which means that they are more efficient because there's more work for biological systems to use. Irreversible reactions are useful for speeding up some other reactions though which is a benefit of them being fast. However, reversible reactions do more wo...
- Sun Feb 07, 2021 11:06 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: delta u = delta H - P delta V
- Replies: 3
- Views: 2220
Re: delta u = delta H - P delta V
The equation delta U = delta H - P*deltaV represents the equation for change in internal energy, demonstrating that deltaH is enthalpy change and -P*deltaV is the equation for work. Yes, this equation can be rearranged to find a missing variable. If you were given the change in internal energy and w...
- Sat Feb 06, 2021 11:47 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: isolated systems and constant energy
- Replies: 6
- Views: 361
Re: isolated systems and constant energy
Since isolated systems do not exchange heat or energy with their surroundings, this is why energy is held constant, and since its value does not change, delta U is 0. An example could be a bomb calorimeter, since a bomb calorimeter is an example of an isolated system that cannot exchange energy with...
- Mon Feb 01, 2021 2:15 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Constant Pressure in Open Systems
- Replies: 8
- Views: 276
Constant Pressure in Open Systems
In today's lecture (02/01), it was mentioned that for the problem with the exothermic reaction (deltaH= -50kJ), an open beaker means constant pressure, but I was wondering how we could arrive at this conclusion. I thought that open beakers would engage more with the surroundings which can affect pre...
- Mon Feb 01, 2021 1:57 pm
- Forum: Calculating Work of Expansion
- Topic: Application behind Infinite Number of Steps and Integral
- Replies: 1
- Views: 80
Application behind Infinite Number of Steps and Integral
I am still a bit confused on the application of where the infinite number of steps applies to in the integral equation for w. Why does a infinite number of steps mean that there is a reversible change?
- Mon Feb 01, 2021 1:52 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Lecture, delta P for aqueous solution editing gas
- Replies: 4
- Views: 174
Re: Lecture, delta P for aqueous solution editing gas
I believe it should be the change in the volume of the solution because that is the system that we are measuring the initial volume from, and then when the gas is emitted, we measure the final volume to recognize the difference after the reaction.
- Mon Feb 01, 2021 1:51 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: reversible process
- Replies: 3
- Views: 109
Re: reversible process
You can also identify that a process is reversible if the internal pressure and external pressure are equal.
- Mon Feb 01, 2021 1:39 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible versus Irreversible Equation
- Replies: 4
- Views: 112
Reversible versus Irreversible Equation
Hi, I was wondering if the equation that was used in lecture today, w = -PdeltaV, is for irreversible or reversible changes in the system?
- Mon Feb 01, 2021 9:57 am
- Forum: Calculating Work of Expansion
- Topic: Negative Sign in w = -PdeltaV
- Replies: 5
- Views: 278
Negative Sign in w = -PdeltaV
I was just wondering if there will always be a negative sign in the equation w = -PdeltaV, since I know that expansion is work done by the system and thus should be negative, whereas compression is work done on the system and therefore work would be positive. Would it always work out if the negative...
- Thu Jan 28, 2021 4:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Exercise 5G.2 (b)
- Replies: 1
- Views: 95
Textbook Exercise 5G.2 (b)
I was doing the exercise 5G.2 part (b) in the textbook, which is a True or False statement saying: The equilibrium concentrations will be the same whether one starts with pure reactants or pure products. I wasn't sure if this was true or false because I know that it can still reach equilibrium, but ...
- Mon Jan 25, 2021 3:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive Property versus Intensive Property
- Replies: 5
- Views: 300
Extensive Property versus Intensive Property
I was wondering what the difference between an extensive property and an intensive property is. I was not sure why extensive properties are not useful as opposed to intensive properties such as specific heat capacity and molar heat capacity. Could anyone explain this?
- Mon Jan 25, 2021 3:16 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity verus Specific Heat Capacity and Molar Heat Capacity
- Replies: 3
- Views: 160
Heat Capacity verus Specific Heat Capacity and Molar Heat Capacity
In lecture today, it was mentioned that there are three terms: heat capacity, specific heat capacity, and molar heat capacity. Will we ever be using heat capacity, or are we going to be focusing more on specific heat capacity and molar heat capacity? Do these differences arise from extensive propert...
- Mon Jan 25, 2021 3:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Monday's lecture clarification
- Replies: 3
- Views: 161
Re: Monday's lecture clarification
Hi, I believe the -2.9 kJ value is different from the -2900 J value because of the units. -2900 J must have been converted into kilojoules by dividing by 1000. In the equation, the value for specific heat capacity that was used was in J/degrees C x g, which would result in a Joules answer for q, but...
- Mon Jan 25, 2021 3:09 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Perfect System
- Replies: 5
- Views: 224
Re: Perfect System
We should not assume that there is a perfect system, but in the concepts and the problem that we solved in lecture, using the concept of a perfect system allowed us to make the connection for the equation q(system) + q(surroundings) = 0, because this equation shows that whatever heat is absorbed by ...
- Mon Jan 25, 2021 3:06 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 1/25 Lecture Example Question
- Replies: 5
- Views: 294
Re: 1/25 Lecture Example Question
The temperature used in the equation is +6.9 degrees Celsius because in the equation for q, which is mass(specific heat capacity)(deltaT), delta T is involved. Delta T is the change in temperature, so it is final temperature minus initial temperature. In the problem, we are given that the final temp...
- Mon Jan 25, 2021 12:10 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6D.15 and 6D.17
- Replies: 3
- Views: 219
Re: 6D.15 and 6D.17
For questions 6D.15 and 6D.17, you need to refer to Tables 6C.1 and 6C.2 to find Ka or Kb values. You would definitely need the Ka or Kb values in order to solve these problems. For instance, in 6D.15 part (a), you can see that Cl- is a spectator ion, and thus the acid NH4+ is concerned. You can obt...
- Sun Jan 24, 2021 6:09 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gases and Le Chatelier's Principle
- Replies: 6
- Views: 518
Inert Gases and Le Chatelier's Principle
If you had a reaction with an inert gas in it, and you increase the partial pressure of that inert gas, does that affect the equilibrium at all? Like if the inert gas was a reactant, would the equilibrium shift to the products or would there be no change?
- Thu Jan 21, 2021 11:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka1 vs Ka2
- Replies: 6
- Views: 1263
Ka1 vs Ka2
What is the rule about Ka1 and Ka2 when doing acid equilibrium problems? I read something about Ka2 being less than Ka1, but I'm not sure where this applies and what it is used for. Could you explain the significance of Ka1 compared to Ka2?
- Thu Jan 21, 2021 11:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Polyprotic Acid Assumptions
- Replies: 1
- Views: 75
Polyprotic Acid Assumptions
When given a polyprotic acid, do we have to assume that we need to calculate equilibrium concentrations at the last step when the acid has given off as many H+ as possible? I'm just not sure what to do when I see a polyprotic acid and have to find a value such as pH, since I do not know whether to j...
- Wed Jan 20, 2021 9:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 6A 21
- Replies: 4
- Views: 170
Re: Textbook Problem 6A 21
Since it was stated that the temperature is 37 degrees, you would be able to recognize that the Kw value would not be the 1.0 x 10^-14 that we have seen. The value for Kw that is 1.0 x 10^-14 applies to when the temperature is 25 degrees.
- Wed Jan 20, 2021 9:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: HW Question
- Replies: 3
- Views: 171
Re: HW Question
In addition to what has been explained regarding how to solve the problem, it is also important to remember that you need to use an ICE box because it has been stated that a weak acid is involved, rather than a strong acid. Thus, not all of the weak acid completely dissociates, so an ICE box would b...
- Wed Jan 20, 2021 5:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Intermolecular Forces during Phase Changes
- Replies: 4
- Views: 296
Intermolecular Forces during Phase Changes
When bonds are being broken or being formed during phase changes, are these intermolecular forces London Dispersion forces? I know Dr. Lavelle mentioned the hydrogen bonds being re-formed during condensation, but I wasn't sure if it is just Hydrogen bonds or just London Dispersion forces or a combin...
- Wed Jan 20, 2021 5:57 pm
- Forum: Phase Changes & Related Calculations
- Topic: Question about Steam Causing Severe Burns
- Replies: 3
- Views: 256
Question about Steam Causing Severe Burns
Hi, I was just wondering if steam coming into contact with skin results in condensation. When the water vapor hits a skin surface, does the vapor immediately turn into liquids? Are those hydrogen bonds immediately broken? I was just wondering about this event conceptually. Thank you in advance!
- Wed Jan 20, 2021 5:55 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Change
- Replies: 5
- Views: 342
Re: Phase Change
I think that a state itself might not be a state property, since state properties usually describe conditions at that state, such as temperature and pressure. A state might just be the end result of a pathway, and the conditions surrounding it would be the state properties. From one state to another...
- Tue Jan 19, 2021 7:43 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Textbook Problem 6B.5 part e
- Replies: 3
- Views: 183
Re: Textbook Problem 6B.5 part e
When you get the value of pOH, use the equation 14 = pOH + pH, and solve for pH by using pH = 14 - pOH.
- Tue Jan 19, 2021 7:41 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Textbook 6D.5
- Replies: 3
- Views: 219
Re: Textbook 6D.5
Yes, in this case you would need the Ka/Kb values or the pKa/pKb values in order to solve these problems. They give it to you in a table in the section as it will help you identify which values you need and help you practice writing the correct equations with the correct products.
- Sun Jan 17, 2021 9:26 pm
- Forum: Ideal Gases
- Topic: Kp?
- Replies: 30
- Views: 1034
Re: Kp?
Yes, as partial pressure can only be measured for gases. With Kc, you are looking at the concentrations of substances, in which case you would omit solids and liquids.
- Sat Jan 16, 2021 9:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Question 6E.1
- Replies: 2
- Views: 136
Textbook Question 6E.1
For question 6E.1 in the textbook, it asks:
Calculate the pH of 0.15 M H2SO4(aq) at 25 degrees C.
How do we know that we have to write out an ICE box for this table and deprotonate H2SO4? I originally thought that you could just take -log of 0.15 M because H2SO4 is a strong acid. Thanks in advance!
Calculate the pH of 0.15 M H2SO4(aq) at 25 degrees C.
How do we know that we have to write out an ICE box for this table and deprotonate H2SO4? I originally thought that you could just take -log of 0.15 M because H2SO4 is a strong acid. Thanks in advance!
- Sat Jan 16, 2021 9:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Question 6D.15 (b)
- Replies: 1
- Views: 127
Textbook Question 6D.15 (b)
Hi, I was wondering about question 6D.15 part (b) in the textbook, which states: Calculate the pH of (a) 0.19 M NH4Cl(aq); (b) 0.055 M AlCl3(aq) My question is how do you write the chemical equation for AlCl3, and how do you find the Ka value? I could not find any Ka values in the textbook tables th...
- Mon Jan 11, 2021 2:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ba(OH)2 example
- Replies: 8
- Views: 955
Re: Ba(OH)2 example
[H3O+] was not included in the equation because when Ba(OH)2 dissociates, it only dissociates into Ba2+ and OH-. Ba2+ is a spectator ion, so it does not affect our calculations. Then, you can use stoichiometry to see that for 1 mole of Ba(OH)2, there are 2 moles of OH-. Thus, we multiply the concent...
- Mon Jan 11, 2021 2:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium constant for water
- Replies: 4
- Views: 335
Re: Equilibrium constant for water
When water is neutral, that means the concentration of H3O+ and OH- are equal. Since Kw has to be constant, [H3O+] and [OH-] have to be the same value to equal Kw, which is 1.0 x 10^-14. Thus, [H3O+] = 1.0 x 10^-7 and [OH-] = 1.0 x 10^-7.
- Mon Jan 11, 2021 2:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw in Lecture
- Replies: 5
- Views: 361
Re: Kw in Lecture
Since Ba(OH)2 is a strong base, Ba2+ is a spectator ion. Spectator ions end up being cancelled out, so that is why it is not included in the Kw equation.
- Mon Jan 11, 2021 2:05 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: ICE Table
- Replies: 9
- Views: 443
Re: ICE Table
For the concept of Le Chatelier's Principle, a reaction that is not at equilibrium is going to want to achieve an equilibrium state, which means that the reactants are going to be used up and the products are going to form. Since reactants are being used up, the change in their concentration is decr...
- Mon Jan 11, 2021 11:10 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Textbook problem 5.61
- Replies: 3
- Views: 111
Re: Textbook problem 5.61
Hi, if the system is compressed, you are increasing pressure and decreasing volume, so you are right! After establishing this, you can look at the number of moles of gas on each side of the equation. Aqueous solutions would not be included in this observation.
- Sun Jan 10, 2021 8:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: identifying solids and non homogeneous reactant/product
- Replies: 3
- Views: 117
Re: identifying solids and non homogeneous reactant/product
Usually in the equation, the phases are stated next to the reactants and products. Solids are identified as (s), liquids as (l), aqueous solutions as (aq), and gases as (g).
- Sun Jan 10, 2021 7:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q and K
- Replies: 8
- Views: 413
Re: Q and K
K is the value for the equilibrium constant, which means that it is a value that is specific to when a reaction is at equilibrium. However, Q is the reaction quotient to represent the ratio of products to reactants when the reaction is not at equilibrium. You can compare the value of Q to the value ...
- Sun Jan 10, 2021 10:26 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Are there reactions that just do not/ can not ever reach equilibrium?
- Replies: 18
- Views: 1773
Re: Are there reactions that just do not/ can not ever reach equilibrium?
Every chemical reaction should have the potential to reach equilibrium given that it has the right conditions to reach the reaction's equilibrium constant. This could depend on things like time, since reactions need time to occur and reach that equilibrium constant.
- Sun Jan 10, 2021 10:24 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sign of x in ICE Box
- Replies: 8
- Views: 432
Re: Sign of x in ICE Box
Reactants would have a negative sign and products would have a positive sign for x since reactants are being used up and their change is decreasing, while products are being produced and their change is increasing.
- Sun Jan 10, 2021 10:23 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Fall 2010, Question #6C (Equilibrium shifts right or left?)
- Replies: 4
- Views: 514
Re: Fall 2010, Question #6C (Equilibrium shifts right or left?)
If you increase the concentration of products at equilibrium, then that means the equilibrium constant needs to remain constant. Since the number in the numerator is now increased, by Le Chatelier's Principle, we need to increase the number in the denominator too, which represents the concentration ...
- Sun Jan 10, 2021 10:20 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5I.19
- Replies: 2
- Views: 105
Re: Textbook Problem 5I.19
Since 60% of the hydrogen gas reacted, this means that there is 40% left at equilibrium, so you would have 40% of the initial concentration of hydrogen gas left at equilibrium (which is stated in the problem as 0.400 mol/3.00 L) and thus calculate it from there using the equilibrium table.
- Sun Jan 10, 2021 10:18 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium constant between 10^-3 and 10^3
- Replies: 6
- Views: 687
Re: Equilibrium constant between 10^-3 and 10^3
I know that specifically when the equilibrium constant is 0, we would not see that because of how that would mean the product concentration would have to be 0 at equilibrium, which is not reasonable.
- Wed Jan 06, 2021 6:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Net Ionic Equations
- Replies: 4
- Views: 139
Net Ionic Equations
When given a reaction with aqueous reactants or products, do we need to first write out the net ionic equation in order to write out the K expression? Or do we just leave the aqueous reactants and products in their original form? Thank you in advance!
- Mon Jan 04, 2021 5:52 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Concentration Amounts of Reactants and Products
- Replies: 6
- Views: 418
Concentration Amounts of Reactants and Products
I was wondering, if a reaction is not at equilbirium, does that mean the concentrations of a reactant and a product would be different between the forward and reverse reactions? I know in lecture today Dr. Lavelle mentioned that it doesn't matter if you are looking at the forward reaction of a syste...
- Mon Jan 04, 2021 1:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How do you write the K for an equation with heterogeneous equilibria?
- Replies: 10
- Views: 808
Re: How do you write the K for an equation with heterogeneous equilibria?
You would just not include the solid as is the standard procedure for writing out the equation. Both solids and liquids are not included in the equilibrium constant. You would, however, include the gas and aqueous solution.
- Mon Jan 04, 2021 12:37 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Chem Equilibrium Pt.4 Post-Assess
- Replies: 4
- Views: 136
Re: Chem Equilibrium Pt.4 Post-Assess
Anytime that the given heat is negative, you would identify this as an exothermic reaction because heat is being given off. Thus, if heat is being given off, or produced, this means that heat is a product. An increase in the products means that to return to equilibrium, a shift to the left, or a shi...
- Mon Jan 04, 2021 11:59 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Part 3 Post-Assessment #19
- Replies: 2
- Views: 139
Chemical Equilibrium Part 3 Post-Assessment #19
For number 19 in the Chemical Equilibrium Part 3 Post-Assessment, the question asks: 0.482 mol N2 and 0.933 mol O2 are placed in a 10.0 L reaction vessel and form N2O (dinitrogen oxide): 2 N2(g) + O2(g) ⇌ 2 N2O(g) KC = 2.0 x 10^-37 What is the composition of the equilibrium mixture? I understand how...
- Mon Jan 04, 2021 11:53 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs. Kc/Kp
- Replies: 15
- Views: 508
Re: K vs. Kc/Kp
I think that the use of Kc and Kp is there to clarify if you are dealing with concentration or pressure, respectively, whereas K is just a broad term for the reaction.
- Mon Jan 04, 2021 11:51 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Sapling #7
- Replies: 6
- Views: 252
Re: Sapling #7
Oh that makes sense! Thank you! Just a quick question, though, so why does Q not change even though more products are being produced? I was just confused by how the reactant/product concentrations stayed the same since we're looking at the reaction immediately after an increase in temperature and m...
- Mon Jan 04, 2021 11:43 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Sapling #7
- Replies: 6
- Views: 252
Re: Sapling #7
Hi, Note, it is important to remember the equilibrium constant K is dependent only on temperature. Changes to initial conditions such as initial concentrations, initial partial pressures, or introductions of catalysts do not affect the value of K . When the temperature is increased for the system, ...
- Mon Jan 04, 2021 11:41 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Sapling #7
- Replies: 6
- Views: 252
Re: Sapling #7
The only time that K is able to change is with a change in temperature. The problem asks about what happens immediately after an increase in temperature. This means that the reactants/products have not had a chance to respond yet, and their concentrations remain the same as before the increase in t...
- Mon Jan 04, 2021 11:22 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Sapling #7
- Replies: 6
- Views: 252
Sapling #7
Hi, I was confused about the reaction quotient component of number 7 from Sapling. The question gives an endothermic reaction and asks "What can be said about Q and K immediately after an increase in temperature?" I understood the second part of the question in which the system would shift...
- Sat Dec 12, 2020 10:45 pm
- Forum: Hybridization
- Topic: Pi Bonds and Hybrid Orbitals
- Replies: 1
- Views: 167
Pi Bonds and Hybrid Orbitals
If you draw out the Lewis structure for CO2 and find the hybrid orbitals, I get that the sigma bond for the bond between C and O is (C 2sp, O 2sp^2). But for the pi bond, would it be (C 2p^2, O 2p)? I want to make sure that this carbon part of the pi bond is correct because if carbon is sp hybridize...
- Mon Dec 07, 2020 4:57 pm
- Forum: Bronsted Acids & Bases
- Topic: Sapling #6 week 10
- Replies: 4
- Views: 386
Re: Sapling #6 week 10
One of the methods that helps me initially determine if an acid is strong or weak is by referring to the seven strong acids that can be found in the textbook. Since HCN is not one of them, that helps me identify it as a weak acid.
- Mon Dec 07, 2020 4:54 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Electronegativity/acidity
- Replies: 5
- Views: 330
Re: Electronegativity/acidity
Electronegativity is important when considering acidity because the more electronegative an element is, the more electron pulling power it has and thus it can distort a negative formal charge from atoms such as an oxygen in a molecule. Thus, this pull from a highly electronegative element is able to...
- Mon Dec 07, 2020 4:49 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric Compounds
- Replies: 4
- Views: 567
Re: Amphoteric Compounds
Yes, both of them can be considered amphoteric because they each have a H+ and a negative charge. This means that they can act as an acid by donating an H+ or act as a base by accepting an H+ and becoming a neutral compound.
- Mon Dec 07, 2020 4:47 pm
- Forum: Bronsted Acids & Bases
- Topic: Group 1 and 2 Cations
- Replies: 3
- Views: 193
Group 1 and 2 Cations
Just to make sure, are cations from Group 1 and 2 acids? I know that they do not affect pH, and more so for Group 1, but it was mentioned today that the cations Fe3+, Cr3+, Al3+, Cu2+, and Ni2+ act as Lewis acids. What is the importance of this statement?
- Mon Dec 07, 2020 2:43 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Effect of pKa on Proton Being Accepted/Donated
- Replies: 2
- Views: 115
Effect of pKa on Proton Being Accepted/Donated
It was discussed that when the solution is more basic as the pH is greater than the pKa, then the weak acid gives off a proton. Is the basic solution itself the weak acid that is being referred to? I thought that bases accept protons, so I'm slightly confused on this.
- Mon Dec 07, 2020 2:31 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: pH and pKa Relation
- Replies: 3
- Views: 227
pH and pKa Relation
I'm still a bit confused on the pH and pKa example about the biological acid, HA, from today's lecture. Are pH and pKa the same concept describing one acid? Or are there two different acids in this example? Could you clarify the molecules involved and why we have these two different values? Thank you!
- Sun Dec 06, 2020 7:04 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Dentates
- Replies: 7
- Views: 354
Re: Dentates
You can find the dentate number by looking to see how many lone pairs are available in a ligand to bind with a transition metal. For example, if there is only one lone pair that can be donated, then it is a monodentate. Additionally, if there were 2 lone pairs available to be donated, then the liga...
- Wed Dec 02, 2020 11:50 pm
- Forum: Bronsted Acids & Bases
- Topic: Compounds Becoming Acids
- Replies: 1
- Views: 160
Compounds Becoming Acids
In today's lecture, Dr. Lavelle mentioned the -COOH groups in the biological molecules. I was just wondering, when he says that these compounds need to be in the presence of water, does that mean that the entire compounds are not acids until they are placed in water and thus the hydrogen is donated ...
- Tue Dec 01, 2020 12:27 am
- Forum: Naming
- Topic: Order of Molecules
- Replies: 6
- Views: 376
Order of Molecules
I was doing a UA worksheet when I saw that we had to write the formula for this coordination compound: Diamminedichloronickel. It is supposed to be [NiCl2(NH3)2], but does anyone know why the Cl2 is before the (NH3)2? I was going in the order of the name, but why are these two switched inside the br...
- Mon Nov 30, 2020 4:10 pm
- Forum: Biological Examples
- Topic: Effects of Partial Pressure
- Replies: 1
- Views: 131
Effects of Partial Pressure
In today's lecture, Dr. Lavelle mentioned both partial pressure and pH as having effects on oxygen. I understand that if pH is changed, this could have an effect on shape and thus the binding of oxygen, but what is the effect of changing partial pressure? Could you explain why oxygen transport and t...
- Mon Nov 30, 2020 4:08 pm
- Forum: Biological Examples
- Topic: Porphyrin Ligand
- Replies: 3
- Views: 175
Porphyrin Ligand
Could someone explain the importance of the porphyrin ligand? I'm not sure what its components are in relation to the myoglobin structure. Is it describing all four of the nitrogen molecules, or something else? Thank you!
- Mon Nov 30, 2020 4:02 pm
- Forum: Biological Examples
- Topic: Biological Functions of Transition Metals
- Replies: 6
- Views: 339
Biological Functions of Transition Metals
I was wondering if the order of transition metals in the periodic table has anything to do with their characteristics for biological functions. Dr. Lavelle mentioned today that chromium was one of our earlier exceptions for electron configurations when he was mentioning its biological function, and ...
- Mon Nov 30, 2020 3:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number of Metal Species
- Replies: 2
- Views: 164
Re: Coordination Number of Metal Species
The coordination number is simply the number of bonds that are attached to the transition metal. In an example used in lecture today, [Ni(CN4)]2- has a coordination number of 4.

