Search found 122 matches
- Sun Mar 14, 2021 5:15 pm
- Forum: Administrative Questions and Class Announcements
- Topic: THANK YOU DR LAVELLE!
- Replies: 47
- Views: 6659
THANK YOU DR LAVELLE!
SHOUTOUT TO DR. DJ LL for a fantastic time in Chem14A/14B. Many of us wanted to be able to show our appreciation in a special way, so we hope you enjoy the following: https://docs.google.com/presentation/d/1q-E6tOSfc_ebMBN4sPMF7VGNyqXz8T2pr1qh7vIK0uw/edit?usp=sharing Thank you everyone for a great t...
- Sun Mar 14, 2021 5:11 pm
- Forum: Student Social/Study Group
- Topic: THANK YOU DR LAVELLE!
- Replies: 2
- Views: 587
THANK YOU DR LAVELLE!
SHOUTOUT TO DR. DJ LL for a fantastic time in Chem14A/14B. Many of us wanted to be able to show our appreciation in a special way, so we hope you enjoy the following: https://docs.google.com/presentation/d/1q-E6tOSfc_ebMBN4sPMF7VGNyqXz8T2pr1qh7vIK0uw/edit?usp=sharing Thank you for a great time learn...
- Fri Mar 12, 2021 8:23 pm
- Forum: Student Social/Study Group
- Topic: Game Night! + **more
- Replies: 1
- Views: 185
Game Night! + **more
Hi everyone! We've been thinking about holding a post finals game night when people are free of obligaiton to just meet others in our fantastic Chem14B//Lavelle's student (Chemistry) community. If you have any suggestions for games to play or days you would be free over the Spring Break, please resp...
- Wed Mar 10, 2021 2:06 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Intermediates? [ENDORSED]
- Replies: 4
- Views: 337
Reaction Intermediates? [ENDORSED]
How are reaction intermediates determined for when we discuss reaction mechanisms? Do we need to know that or will that be given?
- Sun Mar 07, 2021 10:08 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Focus Exercise 6.61
- Replies: 2
- Views: 371
Re: Focus Exercise 6.61
Hi Stella! Conceptually, neurons send off signals based on the strength of the difference in charge of ions inside and outside the cell. In terms of chemistry, we're only looking at the potential based on the potassium ions to cause a reaction. We can assume that, since we are only looking at potass...
- Sun Mar 07, 2021 9:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Problem 6L.9
- Replies: 2
- Views: 249
Re: Textbook Problem 6L.9
I believe this iron reaction may have been chosen to complete the half reaction with potassium permanganate since Fe2+ to 3+ loses another electron (oxidized) while Fe2+ to Fe gains electrons (reduction occurs). The opposite of what happens with the potassium permanganate reaction should occur, and ...
- Sun Mar 07, 2021 9:46 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Explanation of Reaction Progress
- Replies: 3
- Views: 264
Re: Explanation of Reaction Progress
Hi Ashley! This is reviewd in section 7D.2 on collision theory in the textbook. Collision theory reviews how kinetic and potential energy are balanced are particles collide during a reaction. I've attached two reaction profiles that, not only help indicate activation energies, but show the relations...
- Sun Feb 28, 2021 10:47 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #18
- Replies: 3
- Views: 206
Sapling Week 7/8 #18
Hello, I was wondering what the issue is with this eqution? In my calculations, the elements are all balanced. Also, I was wpmderomg of anyone could explain hydrates?
- Sun Feb 28, 2021 10:05 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Wk 7/8 #13
- Replies: 2
- Views: 215
Sapling Wk 7/8 #13
I'm confuseda bout #13 since finding the reduction potentials did not really help me determine the oxidizing agents? Does anyone understand how to determine which reagents can vs cannot oxidize, and what makes one reagent better to oxidize one compound rather than another?
- Sun Feb 28, 2021 6:54 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling wk 7/8 #5
- Replies: 2
- Views: 223
Sapling wk 7/8 #5
For the second half of the problem, I was given N2H4 + ClO3 --> NO + Cl-. When I write the half reactions and add them up, however, I get electrons on the same side (they don't cancel?)
Here is what I have so far:
8e- + 3H2O + N2H4 --> 2NO + 8H+
6e- + 6H+ + ClO3 -- Cl- + 3H2O
Here is what I have so far:
8e- + 3H2O + N2H4 --> 2NO + 8H+
6e- + 6H+ + ClO3 -- Cl- + 3H2O
- Sun Feb 21, 2021 10:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E Cell Equation
- Replies: 2
- Views: 162
Re: E Cell Equation
Hi!
Stuti is right; remember that there are electrolytes in solution, so you need to consider when the ions at the actual electrodes are released into solution. Not sure if I can go more in depth that Sturti did (nice job haha) but keep this in mind!
Stuti is right; remember that there are electrolytes in solution, so you need to consider when the ions at the actual electrodes are released into solution. Not sure if I can go more in depth that Sturti did (nice job haha) but keep this in mind!
- Sun Feb 21, 2021 10:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Oxidation & Reduction
- Replies: 3
- Views: 234
Re: Oxidation & Reduction
I was taught "OIL RIG" in high school: "oxidation is loss, reduction is gain" (in terms of electrons). Hope that helps!
- Sun Feb 21, 2021 10:46 pm
- Forum: Phase Changes & Related Calculations
- Topic: oxidation number change
- Replies: 7
- Views: 641
Re: oxidation number change
Hi! In the context of electrochem, oxidation of a compound causes it to lose electrons. This is important since the oxidation thus drives part of the electric current in a Galvanic cell. It happens at the anode as well. In context of these reactions, you'll need to be able to write the equation for ...
- Sun Feb 21, 2021 10:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cell Significance
- Replies: 2
- Views: 180
Re: Galvanic Cell Significance
Hi! Galvanic Cells as built to drive electrons (so an electric current) through redox reactions between an anode and cathode, and electrolytes in solution that help run those redox reactions. The textbooks says that its basically pushing electrons from one electrode to another. Overall, ionic intera...
- Sun Feb 21, 2021 10:25 pm
- Forum: Balancing Redox Reactions
- Topic: sapling 7/8 number 3
- Replies: 4
- Views: 294
Re: sapling 7/8 number 3
I believe the basic solution just is an indicator of hydroxide ions and "spectator" ions present in solution!
- Wed Feb 17, 2021 10:10 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Sapling wk 5-6 #8
- Replies: 1
- Views: 164
Sapling wk 5-6 #8
Why do we need to calculate the entropy when water boils and cools down to find standard molar entropy of vaporization at a particular temperature?
- Sun Feb 14, 2021 11:20 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook Problem 4A.5
- Replies: 2
- Views: 231
Re: Textbook Problem 4A.5
Hi! For this problem, you'll need to calculate the work likely betweenthe two scenarios using w=-PdeltaV for the irreversible part. Reversible expansions rely on infinitismal changes, so you would use w=-nRTlnV1/V2 for that part. Overall, it makes sense to complete this with calculations, but use yo...
- Sun Feb 14, 2021 11:15 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4F.1 part a
- Replies: 2
- Views: 194
Re: 4F.1 part a
Hi! I think this might be because the question is asking how much entropy the body is generating when it generates heat, so it might be context dependent? Otherwise, I definitely see the confusion along with the need to include the negative sign.
- Sun Feb 14, 2021 11:13 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Textbook 4A.3
- Replies: 2
- Views: 186
Re: Textbook 4A.3
Hi,
The key I'm looking at shows the work to be 28J and the internal energy change to be 28J as well due to the lack of change in temperature. Not sure which key you are looking at?
The key I'm looking at shows the work to be 28J and the internal energy change to be 28J as well due to the lack of change in temperature. Not sure which key you are looking at?
- Sun Feb 14, 2021 11:09 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 4H.1 part d
- Replies: 4
- Views: 288
Re: 4H.1 part d
Hi! In this case, it makes sense to look at the relationship between pressure and volume because we are looking at two samples of the same element in the same phase and same amount!
- Sat Feb 13, 2021 8:51 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Little Confusion about the Overall Formula [ENDORSED]
- Replies: 1
- Views: 172
Little Confusion about the Overall Formula [ENDORSED]
In Monday's lecture, Dr. Lavelle points out that its hard to use deltaS=q/T to calculate entropy in phase changes when the temperature would remain the same. What is the difference/relationship between that focmula and the nCpln(T1/T2) then in calculating entropy when the temperature has infinitisml...
- Sun Feb 07, 2021 5:36 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 2 CO Molecules (from lecture 12)
- Replies: 1
- Views: 158
2 CO Molecules (from lecture 12)
I was curious about this but during lecture 12, when calculating the degeneracy of 2 CO molecules, we have 2 possible positions and 4 atoms, hence degeneracy is 2^4 = 16. For possible states, are we considering them for each moleculre specifically with the total atoms? And not totally within the sys...
- Sun Feb 07, 2021 5:01 pm
- Forum: Phase Changes & Related Calculations
- Topic: Endo vs. exo & bond strenghts
- Replies: 8
- Views: 392
Re: Endo vs. exo & bond strenghts
I think Gabbi did a [censored] job explaining it, but generally you'll see the strength in bond enthalpies as you'll know how much energy is needed to make or break those compounds. If you do see a reaction, however, that seems to have move bonds formed or broken between compounds, it can give you t...
- Sun Feb 07, 2021 4:56 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: w
- Replies: 12
- Views: 784
Re: w
No, you don't need Avogadro's number to find degeneracy. You will only use Avogadro's number for degeneracy if you are finding the degeneracy of a mole of molecules. Well said here! Just like with the first law of thermodynamics, we need to consider context/all factores, here whether we are looking...
- Sun Feb 07, 2021 4:54 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: delta U
- Replies: 6
- Views: 464
Re: delta U
Hi! Not exactly sure what you are referring to, but deltaU=0 when a system is at equilibrium since there will be no change in internal energy. Quoting from Lecture 12 here, for an isolated suste, (so with constant energy) at equilibrium, we have max degeneracy/max entropy, and there is no more chang...
- Sun Feb 07, 2021 4:46 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook 4D.9
- Replies: 2
- Views: 145
Re: Textbook 4D.9
Hi! It doesn't say in the problem but I believe you have to use the enthalpies of formation of all the compounds to find the enthalpy of the reaction. With that, you can find the energy released for each mole of the fuel compound, then (with dimensional analysis bc kJ/mol and the density g/cm^3, sin...
- Sun Feb 07, 2021 4:29 pm
- Forum: Calculating Work of Expansion
- Topic: Irreversible reactions?
- Replies: 2
- Views: 87
Irreversible reactions?
Why is the expansion of a psiton-sealed container at constant pressure considered irreversible?
- Sun Feb 07, 2021 4:25 pm
- Forum: Student Social/Study Group
- Topic: Culinary Chemistry
- Replies: 239
- Views: 35501
Re: Culinary Chemistry
Also I haven't purchased this myself because cookbooks are expensive and all of mine are gifts, but I think there's some review of this in Samin Nosrat's "Salt Fat Acid HEat." I'm almost curious to see exactly why the combination of these different elements can elevate a dish so much more ...
- Sun Feb 07, 2021 4:22 pm
- Forum: Student Social/Study Group
- Topic: Culinary Chemistry
- Replies: 239
- Views: 35501
Re: Culinary Chemistry
ok this thread is so cute I wish I could subscribe to its updates. But I believe this chemistry also applies for salting water for pasta (adding salt gives it higher boiling point means pasta is cooked at a higher temperature). Also, I watched my brother shatter a glass lid one by running it under c...
- Sun Feb 07, 2021 4:05 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Totaling Entropy and Degeneracy
- Replies: 3
- Views: 230
Totaling Entropy and Degeneracy
Hi, I was just wondering if anyone could explain how adding total entropies is given in Kb ln W1 W2 -- totaling degeneracy is multplying them, but what about the Kb?
- Sun Jan 31, 2021 8:52 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated System
- Replies: 16
- Views: 705
Re: Isolated System
Hi! I posted this on another thread but you can think of a styrofoam calorimeter or a vacuum thermos-- the temperature inside theoretically is to stay constant and not exhange any temperature (heat) with the surroundings, like a hydroflask or cooler. (Of course this isn't entirely true in reality bu...
- Sun Jan 31, 2021 8:49 pm
- Forum: Student Social/Study Group
- Topic: Shoutout to Dr. Lavelle
- Replies: 8
- Views: 529
Shoutout to Dr. Lavelle
This quarter has been so rough but we gotta appreciate the homework extension for this week (on top of the countless resources put out to help, the efficient organization of the class, the reminders to exercise the mind and body, the music at the beginning of lecture). Thank you for making online sc...
- Sun Jan 31, 2021 8:42 pm
- Forum: Calculating Work of Expansion
- Topic: Energy and the System/Surroundings
- Replies: 8
- Views: 291
Re: Energy and the System/Surroundings
Hi! I agree with the above post! Energy can be transferred between a system and its surroundings if, for example, heat is absorbed or lost by a system. A real-life example would be a cooling pack. The cooling pack (the system) feels cold because it is absorbing heat from its surroundings (your hand...
- Sun Jan 31, 2021 8:37 pm
- Forum: Student Social/Study Group
- Topic: Work Life Balance
- Replies: 44
- Views: 1737
Re: Work Life Balance
Hey, I totally understand this sentiment. Last quarter I didn't treat myself well at all and it was extraordinarily hard to self-motivate. I've been trying to be more disciplined by training myself with work timers and accepting I can't get every single thing done on my todo list. I'm also spending ...
- Sun Jan 31, 2021 8:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific Heat Capacity
- Replies: 3
- Views: 275
Re: Specific Heat Capacity
Hi! An intensive property only depends on the type of a substance, not the amount. The specific heat capacity is the amount of heat needed to raise the temperature of a specified amount already, such as 1g or 1kg, by 1 K. Therefore, it will be a consistent value no matter how much of the substance y...
- Sun Jan 24, 2021 8:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 #7
- Replies: 8
- Views: 331
Re: Sapling Week 2 #7
Hello! The ice table considers that NaClO in water dissociates into Na+ and ClO-. Since Na+ cannot form a new compound NaOh in water (bc NaOH strong base, therefore it is considered a "spectator ion"), next we see ClO- ions in water. (ClO- aq). Since these equations are all balanced with o...
- Sun Jan 24, 2021 8:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: derive pKW = pH + pOH [ENDORSED]
- Replies: 3
- Views: 421
Re: derive pKW = pH + pOH [ENDORSED]
I would say you hit the nail right on the head; understand how pKa and pKb are derived, how you can observe the autoprotolysis of water, know that pH + pOH = 14, know the conditions of a solution when comparing pH to pKa/pKb, and be able to apply these in context when looking at acid/base dissociati...
- Sun Jan 24, 2021 8:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Making the Approximating for X: K or % Ionization?
- Replies: 6
- Views: 288
Making the Approximating for X: K or % Ionization?
I was wondering which is a more reliable indicator for maknig the approximation? There's a question on the Sapling homework where the K is very small (<10 to the -3) but the % ionization is greater than 5%. In a case like this, which indicator is better?
- Sun Jan 24, 2021 7:47 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam
- Replies: 33
- Views: 1974
Re: Steam
I wanted to further this question; looking at the phase change diagram, we see that heat is continuously supplied during the "liquid vaporization" stage while the temperature remains the same, meaning that when the steam contacts the skin and begins to condense, not only is it at 100 degre...
- Sun Jan 24, 2021 2:29 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pH vs pKa in Equilibrium
- Replies: 1
- Views: 241
pH vs pKa in Equilibrium
kind of an old content question but how can we determine whether a reaction favors reactants or products based on how the pH compares to the pKa/pKb when lokoing at acid/base dissociation?
- Thu Jan 21, 2021 2:20 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How the pH changes in water with a stong acid or base (but mainly acid)
- Replies: 3
- Views: 219
How the pH changes in water with a stong acid or base (but mainly acid)
In one of last week's lectures Dr. Lavelle mentions that adding a weak acid and calculating the concentration of H30+, the pH of a solution in water does not actually change that much. I was wondering about a similar concept reagrding strong acids and abses. Why is the concentration of H+, for examp...
- Wed Jan 20, 2021 12:00 am
- Forum: Ideal Gases
- Topic: 5I.3
- Replies: 4
- Views: 269
5I.3
Would you use pv=nRT to solve this? You're not given a volume, so would we assume 1 L?
- Sun Jan 17, 2021 3:39 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box method
- Replies: 5
- Views: 1246
Re: ICE Box method
Hello, I am having a bit of difficulty understanding how to use the ice box and was wondering if someone could explain how I would use it in these chemical equilibrium problems? So an ice box is used for weak acids or bases that do not completely dissociate. There are three sections to consider: th...
- Sun Jan 17, 2021 3:38 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box method
- Replies: 5
- Views: 1246
Re: ICE Box method
Hi Bronson, I will try my best to help out here. I learned it as RICE (adding an R for the line with the reaction) but it's essentially the same concept. Remember generally using an ICE table you'll be using concentrations, so be sure you have values in M (mol/L) On the first line, you'll want to wr...
- Sun Jan 17, 2021 3:29 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature
- Replies: 45
- Views: 1431
Re: Temperature
Why does temperature change K and not Q values? or does temperature alter the conditions of the reaction enough that K is different?
If the second is true, why would Q remain constant?
If the second is true, why would Q remain constant?
- Sat Jan 16, 2021 11:17 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's with Solids
- Replies: 5
- Views: 240
Le Chatelier's with Solids
If you have a reaction where a solid and gas react to make a gas, would increasing the amount of solid cause the reaction to favor the products? Or would there be no change?
- Tue Jan 12, 2021 12:08 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Affect of Changing Pressure on K
- Replies: 5
- Views: 247
Affect of Changing Pressure on K
I understand that adding an inert gas to alter pressure of a rxn of gases would not change Kc and therefore by Le Chatelier's the reaction would not change/respond. What about Kp though?
- Tue Jan 12, 2021 12:00 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Unchanged K, Changed Pressure
- Replies: 2
- Views: 128
Unchanged K, Changed Pressure
I understand that changing pressure does not change the equilibrium constant, but I just wanted to think about this conceptually: decreasing the volume increases pressure, which increases the reaction rate. So when we look at chemical reactions (here we have conditions iddeal for an increased amount...
- Sun Jan 10, 2021 6:28 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increasing the Yield of the Product.
- Replies: 5
- Views: 457
Re: Increasing the Yield of the Product.
This (also) follows Le Cathelier's Principle, but basically if you reduce the amout of product currently available, the equilibrium will tend to make more since there is less available. I had an old teacher that thought of this as a seesaw: when in equilibrium, products and reactants are in line. If...
- Sun Jan 10, 2021 6:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Dynamic
- Replies: 4
- Views: 392
Re: Equilibrium Dynamic
Le Chatelier's Principle describes what changes dynamic equilibrium. Beyond temperature, pressure and concentration also affect the state of equilibrium! Here's a helpful link that describes this: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_M...
- Sun Jan 10, 2021 6:20 pm
- Forum: Ideal Gases
- Topic: Difference between real and ideal gas
- Replies: 10
- Views: 1157
Re: Difference between real and ideal gas
Although the last few posts appropriately described the difference between ideal gases and real gases, I wanted to bring up a really simple and useful way to quantify how "ideal" a gas really is -- its compressibility. The compression factor (Z) of a gas can be found by comparing the mola...
- Sun Jan 10, 2021 6:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Kc
- Replies: 9
- Views: 459
Re: K vs Kc
Can you recall which section it came from? If I recall correctly, Kc refers to K when the reaction has molarities (typically aqueous reactants and products) when K is just generally referred to as the equilibrium constant. The textbook says (in 5H.3) "You are free to choose either K or KcKc to ...
- Sun Jan 10, 2021 4:23 pm
- Forum: Ideal Gases
- Topic: Advice on how to navigate Chem Community
- Replies: 17
- Views: 906
Re: Advice on how to navigate Chem Community
I'll second Jason here and say that the search function is also extraordinarily helpful in addition to the quick links and board index. Not only is it good for finding sapling questions, but it's also great for looking up specific content within the subtopics. Hope that (and the various other respon...
- Sun Jan 10, 2021 3:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook problem 5G.5
- Replies: 5
- Views: 139
Re: Textbook problem 5G.5
Just wanted to clarify that at equilibrium the concentrations/pressures are "unchanged" only because the forward and backwards reactions are happening at the same rate, not because the reaction is no longer happening.
- Sat Dec 12, 2020 8:24 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Spectator ions
- Replies: 2
- Views: 285
Re: Spectator ions
You can think of the results of dissolving strong acids in bases. Since strong acids and bases dissociate entirely, their resulting ions in solution wouldn't influence the pH since they won't re-bind to the original OH or H. Other examples could be Br- (from HBr) or K+ (from KOH).
- Sat Dec 12, 2020 8:22 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: London Dispersion Force
- Replies: 4
- Views: 452
Re: London Dispersion Force
What Hannah said-- the larger the surface area between two molecules, the greater opportunities to induce dipoles and have LDF interactions. This makes more sense for linear molecules. If you put two pencils together, they would touch at a greater surface area that if you put two ping pong balls tog...
- Fri Dec 11, 2020 1:30 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Textbook Question 2A19)
- Replies: 2
- Views: 345
Textbook Question 2A19)
Why does the Ni 2+ion have two unpaired electrons?
- Thu Dec 10, 2020 10:51 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Acidic, basic, or neutral?
- Replies: 10
- Views: 1670
Re: Acidic, basic, or neutral?
In the lecture from Wed, Dr. Lavelle uses HCl + NH3 --> NH4Cl and explains that the ammonium cation makes the solution acidic. Why is that? Is it based on conjugates or does NH4 donate a proton in solution?
- Thu Dec 10, 2020 12:25 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Determining Acidic Character: Oxoacids and Polarization?
- Replies: 2
- Views: 196
Re: Determining Acidic Character: Oxoacids and Polarization?
(slightly related but in addition why isn't HF a strong acid?)
- Thu Dec 10, 2020 12:25 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Determining Acidic Character: Oxoacids and Polarization?
- Replies: 2
- Views: 196
Determining Acidic Character: Oxoacids and Polarization?
So on sapling question 11 for this week, the feedback I got after getting it wrong (LOL yall pray for me on this final) was that "stronger oxoacids contain more polarized O-H bonds. The more electronegative the central atom, the more polarized the O-H bond will be." I'm confused on how thi...
- Wed Dec 09, 2020 7:33 pm
- Forum: Ionic & Covalent Bonds
- Topic: Clarifying Correct Covalent and Ionic bond Models [ENDORSED]
- Replies: 1
- Views: 258
Clarifying Correct Covalent and Ionic bond Models [ENDORSED]
I undesrtand that part of correcting the covalent bond understand is knowing about dipole moments and polar covalent bonds, and fully understanding the ionic model takes into account size and sharing of electrons. Is there more to it that is fundamental to understanding the characteristics of both t...
- Tue Dec 08, 2020 1:56 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Question from Monday's Lecture?
- Replies: 1
- Views: 98
Question from Monday's Lecture?
Dr. Lavelle in the lecture said a solution is acidic if pH<pKa. Why is that?
Additionally, it was also mentioned that the organic acid HA in the example, when in acidic solution pH of 6, that the acid is protonated (with the H+). Why does this make the organic acid neutral?
Additionally, it was also mentioned that the organic acid HA in the example, when in acidic solution pH of 6, that the acid is protonated (with the H+). Why does this make the organic acid neutral?
- Sun Dec 06, 2020 7:37 pm
- Forum: Student Social/Study Group
- Topic: chelating compounds- i just thought this was funny
- Replies: 1
- Views: 154
chelating compounds- i just thought this was funny
from https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites_(Inorganic_Chemistry)/Coordination_Chemistry/Complex_Ion_Equilibria/Chelation "The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H.D.K. Drew [J. Chem. Soc., 1920, 117, 1456], who stated: ...
- Sun Dec 06, 2020 7:11 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strengths of Acids: Polarity
- Replies: 6
- Views: 596
Re: Strengths of Acids: Polarity
It's probably safe to assume that stronger acids have weaker and more polar hydrogen-X atom bonds because strong acids are more likely to give off their protons. Wouldn't higher polarity make it a theoretically stronger-weak acid? For example, HI has a more polar bond that HBr but since HBr is bigg...
- Sat Dec 05, 2020 4:28 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate Ligands
- Replies: 3
- Views: 141
Re: Polydentate Ligands
Since a ligand bonds in a chelating complex by donating a lone pair, look for several atoms with one pairs within the same molecule; two lone pairs on the same atom can't bond to the metal atom (lone pair e- repulsion) but otherwise two different Nitrogens with lone pairs, for example, in the same m...
- Sat Dec 05, 2020 4:24 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Textbook Exercise 9C.7
- Replies: 3
- Views: 125
Re: Textbook Exercise 9C.7
To further Lea's response, you want to look for two sites on the ligand that can realistically bond to two sites in the coordination complex- Lea did a good job describing how it's more realistic for the two groups with N that are closer to bond to the same metal in a coordination complex, so consid...
- Sat Dec 05, 2020 3:05 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating pH with H+ or H3O+
- Replies: 3
- Views: 207
Calculating pH with H+ or H3O+
Is it possible for an acidic solution to have a different number of moles of H+ and H3O+? (like if there aren't enough H+ for H20?) Or is this not possible in acidic compounds in aqueous solution.
If so, which would you use to calculate the pH of the solution?
If so, which would you use to calculate the pH of the solution?
- Sat Dec 05, 2020 11:50 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strengths of Acids: Polarity
- Replies: 6
- Views: 596
Strengths of Acids: Polarity
When looking at a list of strong acids, I see polar covalent compounds, but I don't quite understand if strong acids or more or less polarizable than weak acids?
- Sat Dec 05, 2020 11:49 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Oxoacids?
- Replies: 4
- Views: 304
Oxoacids?
What are oxoacids and their application? Are they in organic or inorganic compounds? When are oxoacids considered in the scope of this unit?
- Sat Dec 05, 2020 11:47 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Stability of Anions Considering Strengths of ACids
- Replies: 4
- Views: 731
Stability of Anions Considering Strengths of ACids
When characterizing the strengths of acids, one aspect to consider is resonance. I think this has something to do wih the stability of an anion and delocalizing the negative charges. How do we determine the stability of an anion and how does that correspond with the strength of an acid? How does res...
- Sun Nov 29, 2020 5:42 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling #20
- Replies: 5
- Views: 372
Sapling #20
How do we know that one of the bonds with the As and O atoms in the As)4(3-) molecule has to be a double bond? How can I recognize this trend in further mleculres? Is it just bc the As is the central atom?
- Sun Nov 29, 2020 4:47 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Tips on remembering shapes based on atoms in structure?
- Replies: 3
- Views: 262
Tips on remembering shapes based on atoms in structure?
I was thinking Quizlet haha but I want to have a better understanding than just going off of memorization; what's a tip to remember the structures and angles of molecules? I find myself referring to a table I printed that shows the atoms around the central atom and their shapes+angles, but I have so...
- Sun Nov 29, 2020 4:44 pm
- Forum: Sigma & Pi Bonds
- Topic: Rotation on the Internuclear Axis
- Replies: 2
- Views: 677
Re: Rotation on the Internuclear Axis
Hi! The internuclear axis is defined as the straight line connecting the center of the two atoms in a bond. For your first question, the sigma bond does have electron density on both sides of the axis, but the density directly overlaps the axis, as seen in the image below. However, a pi bond is als...
- Sun Nov 29, 2020 4:42 pm
- Forum: Dipole Moments
- Topic: Tetrahedral Atoms and Dipole Moments
- Replies: 7
- Views: 484
Tetrahedral Atoms and Dipole Moments
Will every tetrahedral molecule with different atoms around the central atom have a dipole moment? (ex: CH2Cl2)
- Sun Nov 29, 2020 4:40 pm
- Forum: Resonance Structures
- Topic: Sapling 17 (Specifically Bond Angles)
- Replies: 3
- Views: 280
Sapling 17 (Specifically Bond Angles)
How would you determine the possible bond angles in the various structures? I had difficulty building the different physical structures; how would the three possible structures look in 3D space and how would their angles differ (or really how do the structures of the two non-cylical structures look ...
- Sun Nov 29, 2020 3:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Negative poles of molecules
- Replies: 3
- Views: 278
Re: Negative poles of molecules
How would you determine a molecule's polarity based on the shape if the lewis structure appears to have an equal distribution of the charges? How would you determine the pole through the structure?
- Sat Nov 28, 2020 11:23 pm
- Forum: Sigma & Pi Bonds
- Topic: Rotation on the Internuclear Axis
- Replies: 2
- Views: 677
Rotation on the Internuclear Axis
I'm a bit confused about the diagram of the pi bond forming on the internuclear axis that shows electron density on both sides of the internuclear axis. Doesn't the sigma bond also show electron density on both sides of the internuclear axis? Also, how are pi bonds formed then if not end to end. Las...
- Mon Nov 23, 2020 2:02 am
- Forum: Hybridization
- Topic: Sigma vs Pi Bonds
- Replies: 10
- Views: 395
Re: Sigma vs Pi Bonds
I found this image on Chem Libre texts that might be helpful in visualizing the different bonds visually:
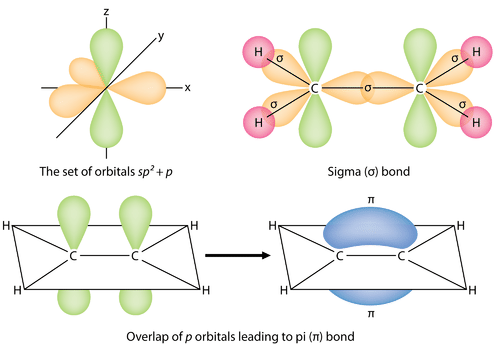

- Mon Nov 23, 2020 1:15 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angle tips
- Replies: 10
- Views: 690
Re: bond angle tips
Theoretically if you know the geometry of a molecule, like how many bonds are in the same plane, you could divide that 360 degrees by that number; honestly, though, it is probably wiser to just use Quizlet to memorize or become familiar with the unique bond angles.
- Mon Nov 23, 2020 1:12 am
- Forum: *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Topic: Viscosity/Surface Tension
- Replies: 7
- Views: 1737
Re: Viscosity/Surface Tension
Viscosity and Surface tension are directly related to one another; increase viscosity then the surface tension increases. This is due to the intermolecular forces between molecules. Take a look at water! Water has surface tension that stems from hydrogen bonding between H20 molecules, this means th...
- Mon Nov 23, 2020 1:07 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Instantaneous Dipoles
- Replies: 5
- Views: 222
Re: Instantaneous Dipoles
If its rod shaped then either end of the rod is likely partially charged and another charged rod can line up antiparallel making the dipole moment at either end of each particle very close to the dipole moments on the other particle. This means that both dipole moments on both molicules are interac...
- Mon Nov 23, 2020 1:04 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Why are hydrogen bonds so strong relative to other dipole-dipole bonds?
- Replies: 11
- Views: 13865
Re: Why are hydrogen bonds so strong relative to other dipole-dipole bonds?
Hydrogen bonding is so strong among dipole-dipole interactions because it itself is a dipole-dipole interaction with one of the strongest possible electrostatic attractions. Remember that hydrogen bonding cannot occur unless hydrogen is covalently bonded to either oxygen, nitrogen, or fluorine. Thi...
- Sun Nov 15, 2020 11:16 pm
- Forum: Ionic & Covalent Bonds
- Topic: Saplong 15
- Replies: 6
- Views: 377
Saplong 15
On Sapling Question 15, why is HF the only compound listed (hydrogen halide) thta remains covalently bonded in water of HF, HBr, HCl, and HI?
- Sun Nov 15, 2020 10:30 pm
- Forum: Bond Lengths & Energies
- Topic: Bonds and Accepting Electrons
- Replies: 2
- Views: 173
Bonds and Accepting Electrons
Sapling Question #6 says 'Molecules with polar double bonds also accept electrons" making CO2 a Lewis Acid. Why is that?
- Sun Nov 15, 2020 10:23 pm
- Forum: Resonance Structures
- Topic: Building Resonance Structures with Similar Formal Charges
- Replies: 2
- Views: 178
Building Resonance Structures with Similar Formal Charges
In building resonance structures with similar distributions of formal charges, how do we determine which is more favorable? I've looked at a few problems with different double/single bonds, same distributions of formal charges, and remember reading somewhere that favorability is influenced by least ...
- Sun Nov 15, 2020 10:12 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Consequences of polarizability
- Replies: 4
- Views: 572
Re: Consequences of polarizability
Since polarizability increases for larger cations, does this mean larger compounds overall have stronger IMFs? Also, how does a dipole moment influence/is influenced by polarizability?
- Sun Nov 15, 2020 9:37 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: dipole moments
- Replies: 10
- Views: 373
Re: dipole moments
Would you only draw these for a dipole moment though? I guess I want to know when you would even draw the arrows; would it be in an ionic compound specifically, or the temporary dipole moment in covalent compounds? Also, I wanted to clarify you wouldn't have a dipole moment in a compound made of the...
- Sun Nov 15, 2020 9:24 pm
- Forum: Bond Lengths & Energies
- Topic: Energies for interactions
- Replies: 4
- Views: 136
Re: Energies for interactions
When reviewing interactoins between ion-ion and ion-dipole interactions, where do the energies come from? I understand that, in an ion-ion example, the two are attracted to each other, but I don't undersatnd how that contributes to the negative energy (being released)? It is better not to think of ...
- Sun Nov 15, 2020 9:20 pm
- Forum: Dipole Moments
- Topic: London Dispersion forces
- Replies: 14
- Views: 580
Re: London Dispersion forces
This might be a silly question but are LDF's, dipole induced dipole, and induced dipole-induced dipole the same? My mind is spinning at hte use of the word dipole over and over ahaha.
- Sun Nov 15, 2020 7:44 pm
- Forum: Bond Lengths & Energies
- Topic: Energies for interactions
- Replies: 4
- Views: 136
Energies for interactions
When reviewing interactoins between ion-ion and ion-dipole interactions, where do the energies come from? I understand that, in an ion-ion example, the two are attracted to each other, but I don't undersatnd how that contributes to the negative energy (being released)?
- Sun Nov 15, 2020 2:56 pm
- Forum: Ionic & Covalent Bonds
- Topic: What do delta positive and delta negative refer to?
- Replies: 3
- Views: 1792
What do delta positive and delta negative refer to?
I know they have to do with the general charge as the electron is puleld between atoms, but I'm not sure specifically what they refer to and how they apply specifically to ionic and covalent bonding-- I did catch how electronegativity is at play there, but still not sure how the delta is involved. A...
- Sun Nov 08, 2020 11:44 pm
- Forum: Resonance Structures
- Topic: Valence Electrons [ENDORSED]
- Replies: 46
- Views: 3911
Re: Valence Electrons [ENDORSED]
Is there a way to use the electron configurations to know the valence electrons in transition metals?
- Sun Nov 08, 2020 11:42 pm
- Forum: Resonance Structures
- Topic: Oxidation Number
- Replies: 9
- Views: 317
Re: Oxidation Number
Does an oxidation number pertain to both loss and gain? How does it relate to reduciton?
- Sun Nov 08, 2020 11:40 pm
- Forum: Octet Exceptions
- Topic: Exceptions
- Replies: 9
- Views: 404
Re: Exceptions
I would agree with what was said above, the way to tell that they are exceptions is that if there is no way for the middle atom to achieve a full octet. You do not need to memorize all of them, I would say its more favorable if you recognize them instead. Is this a way to tell for individual atoms ...
- Sun Nov 08, 2020 11:39 pm
- Forum: Octet Exceptions
- Topic: Exceptions
- Replies: 9
- Views: 404
Re: Exceptions
Is there a way to use either periodic table trends (or indications or orbitals) or just the character of an element to determine of it's an exception to the rule?
- Sun Nov 08, 2020 11:35 pm
- Forum: Lewis Structures
- Topic: Lewis Dot Structure
- Replies: 11
- Views: 433
Re: Lewis Dot Structure
Wouldn't you put one on each side first before you match the pairs of electrons? or am I just thinking of spin... Actually I think this applies since we have to think how the electron alone in an orbital and a pair represents two that are in the same one. Overall you'd probably have to be conscious ...
- Sun Nov 08, 2020 11:04 pm
- Forum: Lewis Structures
- Topic: Bond Character
- Replies: 6
- Views: 301
Re: Bond Character
I believe bond character could also be associated with ionic vs covalent nature which could be related overall to the bond lengths. It could be similar to ionic character, where ions contribute to another molecule or atom's resonance. Replying to follow this post though because I would like a furthe...
- Sun Nov 08, 2020 11:01 pm
- Forum: Ionic & Covalent Bonds
- Topic: London dispersion forces
- Replies: 12
- Views: 802
Re: London dispersion forces
I believe most replies above have the definition of London dispersion forces. Here is the order of bond strengths from strongest to weakest 1. Ionic bonds 2. Covalent bonds 3. Dipole Dipole bonds 4. Van der Waals (London dispersion) Are London Disperson/Van der Waals considered a form of bond? Or a...
- Sun Nov 08, 2020 10:56 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2 Study Group
- Replies: 25
- Views: 1093
Re: Midterm 2 Study Group
Thanks for posing this!
- Sun Nov 08, 2020 10:55 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization and electron affinity
- Replies: 6
- Views: 459
Re: Ionization and electron affinity
Hi, I just wanted to pose a further quesiton; electron affinity involves energy released when an electron is added to an atom; even though it only applies to atoms in their gaseous form, does this essentially hold true with the trend of ionization energy and electronegativity always? Additionally, i...

