Search found 110 matches
- Thu Mar 11, 2021 3:01 pm
- Forum: First Order Reactions
- Topic: Half Life Unit
- Replies: 38
- Views: 1765
Re: Half Life Unit
I think that just looking at what the problem provides you with, should determine what units you are going to use.
- Thu Mar 11, 2021 2:55 pm
- Forum: General Rate Laws
- Topic: Intermediate
- Replies: 59
- Views: 4084
Re: Intermediate
An intermediate or reaction intermediate is a substance formed during a middle step of a chemical reaction between reactants and the chosen product.
- Thu Mar 11, 2021 2:47 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final on 3/13
- Replies: 18
- Views: 1006
Re: Final on 3/13
Like the other comments mentioned, it starts at 9:30 AM this Sunday, but you can join 15 minutes early to get accommodated.
- Thu Mar 11, 2021 2:46 pm
- Forum: General Rate Laws
- Topic: Rate determining step
- Replies: 38
- Views: 1689
Re: Rate determining step
This is because the slow step determines the rate of the reaction. If your eating dinner but can only leave the restaurant after the last person is finished, then they would be considered the slow step. The slow step limits how fast the reaction can proceed.
- Thu Mar 11, 2021 2:40 pm
- Forum: Student Social/Study Group
- Topic: How are y'all doing?
- Replies: 46
- Views: 3007
Re: How are y'all doing?
For me, March went by extremely quickly. I blinked and now it's finals lol. Overall, I would say I am a 7/10. Studying has been a bit of a drag, but honestly, I'm just excited for a break at this point. Also, I definitely agree with the comments about burnout.
- Sun Mar 07, 2021 5:09 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3914643
- Sun Mar 07, 2021 5:03 pm
- Forum: First Order Reactions
- Topic: Slope
- Replies: 24
- Views: 988
Re: Slope
Here's a picture that might be helpful.
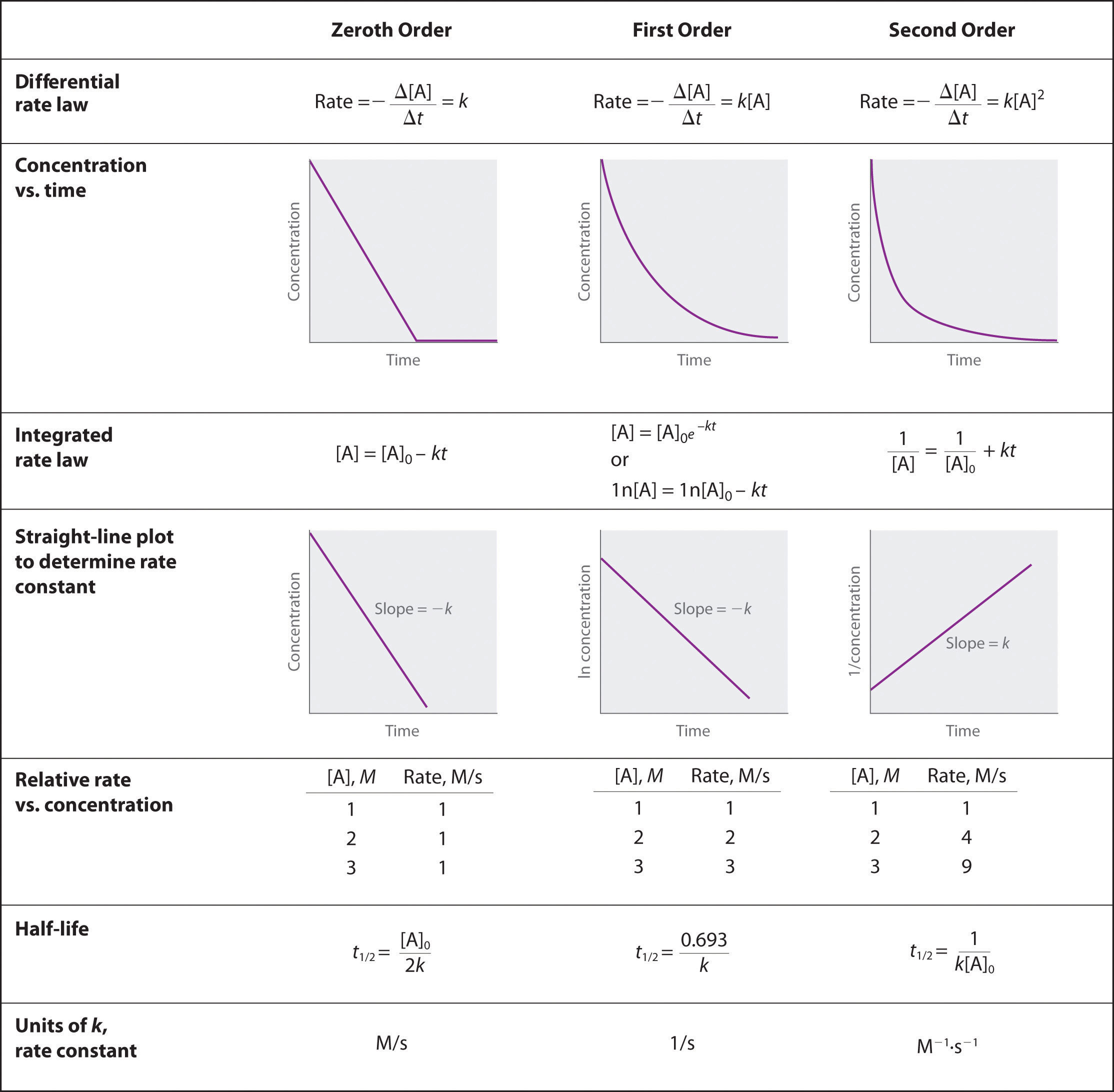

- Sun Mar 07, 2021 4:49 pm
- Forum: Phase Changes & Related Calculations
- Topic: Cp
- Replies: 7
- Views: 515
Re: Cp
I agree with the previous comments. I feel like it would be important for the final so try and include it in your studying.
- Sun Mar 07, 2021 4:39 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 20915
Re: How do you deal with burnout?
I feel like one way to deal with it, is to set goals for the day and give yourself a reward based on what you've accomplished. Also, make sure you have at least one full day a week to yourself, where you do everything but work. That way the next day you'll feel much more renewed.
- Sun Mar 07, 2021 4:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: What is the purpose of having a salt bridge?
- Replies: 16
- Views: 807
Re: What is the purpose of having a salt bridge?
The purpose is to maintain charge balance because the electrons are moving from one-half cell to the other. Without it, the electrons wouldn't be able to flow from the anode to the cathode properly.
- Sun Mar 07, 2021 2:46 pm
- Forum: Administrative Questions and Class Announcements
- Topic: week 10 lectures?
- Replies: 15
- Views: 998
Re: week 10 lectures?
This is a good question. Last quarter I believed we finished around Wednesday, and one of the lectures was a review of the quarter's material. This may happen again, but we have to see what Prof. Lavelle does.
- Sat Feb 27, 2021 7:37 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 30
- Views: 1449
Re: Oxidation Numbers
I agree with the other comments, just practice remembering the common elements and you should be fine. Here's a link to a good review and practice. Hope this helps: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemist...
- Sat Feb 27, 2021 7:28 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Cathodes and Anodes
- Replies: 4
- Views: 303
Re: Cathodes and Anodes
Agreeing, with the above comments they will show you in the diagram, but just remember that the anode is usually on the left and the cathode is on the right, with the anode going through oxidation and the cathode reduction.
- Sat Feb 27, 2021 7:22 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Diamond vs. Graphite
- Replies: 23
- Views: 1166
Re: Diamond vs. Graphite
I agree with the other comments, since the conversion is spontaneous it could happen at room temperature and 1 atm, but it would be a much slower process since the energy being absorbed is low.
- Sat Feb 27, 2021 7:00 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry Community Points
- Replies: 24
- Views: 1259
Re: Chemistry Community Points
Yeah, if you have received 50 points you can still post, you just don't get any extra points since that category is capped at 50.
- Sat Feb 27, 2021 6:54 pm
- Forum: Student Social/Study Group
- Topic: Chem Community Points
- Replies: 35
- Views: 1860
Re: Chem Community Points
The max is 50 points, but you are expected to post at least 5 times a week since there are 5 points per week multiplied by the 10 weeks, hence the total of 50 points.
- Sun Feb 21, 2021 7:21 pm
- Forum: Balancing Redox Reactions
- Topic: Cell/battery
- Replies: 26
- Views: 1069
Re: Cell/battery
Yes, I believe we can use them interchangeably. In Friday's lecture when writing the equation Lavelle used redox, cell, and battery in the E (__potenial), so I'm assuming they represent the same idea.
- Sun Feb 21, 2021 7:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge Purpose
- Replies: 8
- Views: 603
Re: Salt Bridge Purpose
I think the purpose of the salt bridge is that it helps to maintain neutrality between the two sides of the circuit. It's to maintain a charge balance since the electrons are moving from one-half cell to the other. Without it, the ions can't flow between the cathode and anode freely.
- Sun Feb 21, 2021 7:14 pm
- Forum: Student Social/Study Group
- Topic: Fave food
- Replies: 266
- Views: 48151
Re: Fave food
My favorite food is any kind of pasta or sandwich. I've really been into shrimp scampi lately, and also craving mint chocolate chip ice cream.
- Sun Feb 21, 2021 7:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling #6
- Replies: 5
- Views: 429
Re: Sapling #6
Agreeing with the others, I think the battery is set up like this. When Prof. Lavelle told us about E cell= E (Right: cathode) - E (Left: Anode) in Friday's lecture. I think it will always be represented like this.
- Sun Feb 21, 2021 7:04 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: State function?
- Replies: 36
- Views: 1568
Re: State function?
Agreeing with the previous comments, E (cell potential) is not a state function because it is dependent on the path it takes, so it ends up being a path function.
- Sun Feb 14, 2021 6:59 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy vs Entropy
- Replies: 39
- Views: 3361
Re: Enthalpy vs Entropy
This isn't a dumb question at all. Enthalpy has to deal with heat, while entropy has to do with the state of a system. In more depth, enthalpy is the heat content of a system, and enthalpy changes when energy is either lost or gained. Entropy refers to the level of disorder of a system depending on ...
- Sun Feb 14, 2021 6:52 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: temperature
- Replies: 32
- Views: 1468
Re: temperature
Agreeing with most comments, I think the context of the question matters. If the question mentions Kelvins then you should convert like when dealing with entropy. I think it is a good habit to just convert to Kelvin, even if it is not specifically stated because most questions seemed to be formatted...
- Sun Feb 14, 2021 6:44 pm
- Forum: Student Social/Study Group
- Topic: Valentine's Day Long Weekend!
- Replies: 30
- Views: 2317
Re: Valentine's Day Long Weekend!
This is very kind. I hope everyone that reads this post is also able to enjoy their weekend and have a happy Valentine's Day.
- Sun Feb 14, 2021 6:42 pm
- Forum: Calculating Work of Expansion
- Topic: irreversible vs reversible
- Replies: 14
- Views: 757
Re: irreversible vs reversible
If the problem does not directly state it, it might hint by writing "slow" or "quick" expansion or by talking about the type of pressure exerted on the system.
- Sun Feb 14, 2021 6:36 pm
- Forum: Student Social/Study Group
- Topic: Fav Shows of the Moment
- Replies: 115
- Views: 56913
Re: Fav Shows of the Moment
Currently, I've rewatched Game of Thrones, but I have also been watching Queen's Gambit and Scandal. All three shows are fantastic.
- Sun Feb 07, 2021 12:48 am
- Forum: Student Social/Study Group
- Topic: Favorite TV shows
- Replies: 277
- Views: 51491
Re: Favorite TV shows
Just wanted to update:
I've been watching Vampire Diaries, The Walking Dead, Naruto, and Game of Thrones. I've been having a lot of fun watching them.
I've been watching Vampire Diaries, The Walking Dead, Naruto, and Game of Thrones. I've been having a lot of fun watching them.
- Sun Feb 07, 2021 12:45 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong acids/bases
- Replies: 10
- Views: 613
Re: Strong acids/bases
Here's a link to a good website. I hope it helps.
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/16%3A_Acids_and_Bases/16.4%3A_Strong_Acids_and_Strong_Bases
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/16%3A_Acids_and_Bases/16.4%3A_Strong_Acids_and_Strong_Bases
- Sun Feb 07, 2021 12:38 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Standard Entropy
- Replies: 2
- Views: 107
Re: Standard Entropy
Agreeing with Karl, Prof. Lavelle mentioned this in Lecture # 12. The standard enthalpy of vaporization is linked to N or the # number of particles. When a system moves from liquid to gas, the molecules in the gaseous phase occupy more states compared to liquids or solids. This allows more possibili...
- Sun Feb 07, 2021 12:32 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Heat vs Thermal Energy
- Replies: 7
- Views: 292
Re: Heat vs Thermal Energy
I think the main difference is that thermal energy doesn't move and stays inside the system versus heat, which is the energy that can be transferred in and out of a system like with endothermic and exothermic processes.
- Sun Feb 07, 2021 12:24 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Difference in volume and temperature
- Replies: 6
- Views: 599
Re: Difference in volume and temperature
I agree with Andrew and Vince, I think it mainly depends on what is constant when looking at your system. You can also result in the same value too.
- Sun Feb 07, 2021 12:21 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Work on a system
- Replies: 27
- Views: 1233
Re: Work on a system
Just like others have mentioned, the simplest example is the tire pressure example that Prof. Lavelle brought up. Adding air into the tire increases the compression within the tire, which puts work into the system allowing for a positive value for work. Sapling also mentions this on one of their que...
- Sun Jan 31, 2021 8:36 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature Change
- Replies: 16
- Views: 880
Re: Temperature Change
Since the reaction is endothermic that means the system will take in heat. This will cause the reaction to shift right towards the products. Since the system is requiring more heat this will cause K to increase since the numerator will increase.
- Sun Jan 31, 2021 8:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 1
- Replies: 9
- Views: 506
Re: Midterm 1
It usually takes around a week, he also emails us to update how grading is going.
- Sun Jan 31, 2021 8:08 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3914643
Re: Post All Chemistry Jokes Here
If H2O is water and H2O2 is hydrogen peroxide, what is H2O4?
Drinking, bathing, and lots of other activities.
Drinking, bathing, and lots of other activities.
- Sun Jan 31, 2021 8:06 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpies of Formation using Hess's Law
- Replies: 18
- Views: 1189
Re: Standard Enthalpies of Formation using Hess's Law
Yes, you're correct. You can rearrange the equation, but like the other comments mentioned keep track of the sign changes of the enthalpy.
- Sun Jan 31, 2021 7:55 pm
- Forum: Student Social/Study Group
- Topic: Midterm Scores
- Replies: 25
- Views: 1050
Re: Midterm Scores
The professor will send out an email when the grading is close to being finished. So, between a week or two, the grading should be inputted.
- Sun Jan 31, 2021 7:48 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Reset?
- Replies: 17
- Views: 1586
Re: Sapling Reset?
Just as everyone else has mentioned, I don't believe there is a reset button. However, you could copy the question and reanswer them separately, and then check the answer using Sapling. That could be a good method.
- Sat Jan 23, 2021 10:30 pm
- Forum: Phase Changes & Related Calculations
- Topic: bonds and energy
- Replies: 13
- Views: 730
Re: bonds and energy
I believe it has to do with potential energy. Forming bonds releases energy because once they are formed the energy is no longer needed. It gets released which is why it is exothermic, compared to breaking bonds which requires energy.
- Sat Jan 23, 2021 10:21 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Outline 3 Problems
- Replies: 2
- Views: 118
Re: Outline 3 Problems
Agreeing with the post above, he sent out an email listing the specifics of what we should do. It should outline what you should be looking at.
- Sat Jan 23, 2021 10:19 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling vs. Textbook Problems
- Replies: 8
- Views: 486
Re: Sapling vs. Textbook Problems
Speaking from experience, the midterm is much more like the textbook problems. However, Sapling is good at breaking down the solutions step-by-step. Hope this helps.
- Sat Jan 23, 2021 10:07 pm
- Forum: Phase Changes & Related Calculations
- Topic: Vapor vs gas
- Replies: 121
- Views: 15023
Re: Vapor vs gas
Agreeing with everyone else, yes they refer to the same thing. Good question.
- Sat Jan 23, 2021 10:05 pm
- Forum: Phase Changes & Related Calculations
- Topic: Leidenfrost Effect
- Replies: 3
- Views: 266
Leidenfrost Effect
I watched a video of a man touching lava, but very quickly, and yet he doesn't get burned. I looked it up and found out it is related to the Leidenfrost effect. Looking at the heating curve for water, how could we explain this effect?
- Sun Jan 17, 2021 5:48 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure Increase
- Replies: 7
- Views: 395
Pressure Increase
I remember on the post-assessment for module 4, one of the questions mentioned: "State whether the equilibrium will shift toward the products, reactants, or neither." ii. The pressure on the system is increased. The answer was neither. Why is that? I know a change in pressure doesn't chang...
- Sun Jan 17, 2021 5:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ice Box Method
- Replies: 14
- Views: 567
Re: Ice Box Method
Thanks for asking this I had the same question. But, as everyone has mentioned it depends on what the question is asking for and which side the reaction will shift to. The question might say something like "1.50 mol PCl5 is placed..." and you have to look at the equation to see if it is th...
- Sun Jan 17, 2021 5:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemistry Community
- Replies: 4
- Views: 218
Re: Chemistry Community
I think looking at the topic title when clicking on someone's post or if you post might help you differentiate if it is 14A or 14B material. Also, the other post might be right, some people probably just posted in the wrong place. This has never happened to me so I don't have much knowledge of this ...
- Sun Jan 17, 2021 5:14 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: X less than 5 percent
- Replies: 11
- Views: 961
Re: X less than 5 percent
The 5% rule is probably just a guideline. If it is less than 5% you can approximate, but if it is more that is when the quadratic equation comes in. We probably use this rule because anything under 5% is very small, so it doesn't make that much of a difference to the overall composition. For example...
- Sun Jan 17, 2021 5:08 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's Principle and Pressure
- Replies: 6
- Views: 353
Re: Le Chatelier's Principle and Pressure
Just as everyone has mentioned, when there's an increase in pressure, the volume will decrease. When the volume decrease to tell if the reaction will shift either left or right, you have to look at the moles of gas on each side of the equation. If there are more moles on the left then the reaction f...
- Sun Jan 10, 2021 6:38 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Stable Reactants and Products
- Replies: 7
- Views: 256
Re: Stable Reactants and Products
I think stability implies that either the products or reactants are favored. The ratio of P/R is related to the energy difference of R and P. So, with stability depending on K it can tell you whether R or P is favored and the energy difference.
- Sun Jan 10, 2021 6:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Equilibrium Adjustments
- Replies: 10
- Views: 492
Equilibrium Adjustments
I remember it was mentioned in lecture that chemical reactions adjust so as to minimize the effect of changes. What exactly does this mean, how do the reactions adjust themselves?
- Sun Jan 10, 2021 6:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homogeneous and Heterogeneous Equilibrium
- Replies: 13
- Views: 817
Re: Homogeneous and Heterogeneous Equilibrium
Agreeing with others, I believe you look at the overall reaction even if you are only calculating Kc or Kp. So, you look at each phase in the entire reaction and that’s what determines if the equilibrium is homogeneous or heterogeneous.
- Sun Jan 10, 2021 6:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Dynamic
- Replies: 4
- Views: 419
Equilibrium Dynamic
Is there anything else besides temperature that can make an equilibrium dynamic?
- Sun Jan 10, 2021 6:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K=1
- Replies: 8
- Views: 402
Re: K=1
Agreeing with what everyone else said, having both the products and reactants with equal concentrations at equilibrium is very difficult to produce. That’s probably why lavelle mentioned that.
- Tue Dec 15, 2020 12:16 pm
- Forum: Bronsted Acids & Bases
- Topic: lewis vs bronsted
- Replies: 10
- Views: 685
Re: lewis vs bronsted
Here's a good image for simplification. I hope it helps.
- Tue Dec 15, 2020 12:11 pm
- Forum: Student Social/Study Group
- Topic: Winter Break
- Replies: 44
- Views: 2791
Re: Winter Break
I think doing practice problems and just watching some videos will be good over the break. Don't overload yourself, remember you are on break.
- Tue Dec 15, 2020 12:07 pm
- Forum: Student Social/Study Group
- Topic: Staying Motivated
- Replies: 38
- Views: 2025
Re: Staying Motivated
I feel like one way to stay motivated is to set a schedule for yourself and pace yourself. Write out everything you want to accomplish, so you can check it off as you go. Give yourself 15-30 minute breaks every two hours or so, so you don't get fatigued. Also, act like you're still going to class in...
- Tue Dec 15, 2020 12:02 pm
- Forum: Lewis Acids & Bases
- Topic: Classifying Other
- Replies: 4
- Views: 247
Classifying Other
When classifying strong & weak acids, and strong & weak bases, I was curious when do we classify a compound as Other? Just like in the Sapling HW for example.
- Tue Dec 15, 2020 11:57 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating pH help
- Replies: 5
- Views: 426
Calculating pH help
When I was doing the Sapling HW on Sunday I found a really good picture that helped me. I hope it can help anyone that needs it.
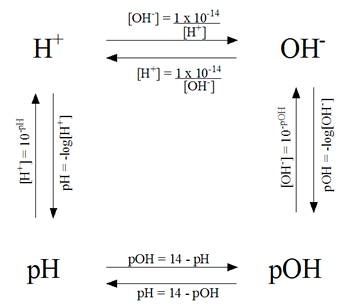

- Sun Dec 06, 2020 1:01 pm
- Forum: Naming
- Topic: Naming Practice
- Replies: 4
- Views: 378
Naming Practice
If anyone needs help naming coordination compounds I found this website extremely helpful:
http://www.chemistry.wustl.edu/~edudev/LabTutorials/naming_coord_comp.html
http://www.chemistry.wustl.edu/~edudev/LabTutorials/naming_coord_comp.html
- Sun Dec 06, 2020 12:53 pm
- Forum: Naming
- Topic: Naming Coordination Compounds
- Replies: 9
- Views: 579
Re: Naming Coordination Compounds
As everyone else mentioned, you only need to add -ate, when the coordination complex has an overall negative charge. Here's a website link that I found was helpful. http://www.chemistry.wustl.edu/~edudev/LabTutorials/naming_coord_comp.html
- Sun Dec 06, 2020 12:33 pm
- Forum: Dipole Moments
- Topic: Dipole canceling
- Replies: 13
- Views: 811
Dipole canceling
So as long as the dipole moments cancel out in a molecule it is considered nonpolar? I was thinking about this because I know bonds can be polar from differences in electronegativity, but based on the shape the molecule can still be nonpolar, right?
- Sun Dec 06, 2020 12:29 pm
- Forum: Biological Examples
- Topic: Complex Ion Biological Importance
- Replies: 3
- Views: 409
Complex Ion Biological Importance
How is a complex ion important for biological processes? Or is it important at all?
- Sun Dec 06, 2020 12:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pair Locations
- Replies: 6
- Views: 462
Lone Pair Locations
How would you explain why lone pairs are more likely to be found in certain locations around a central atom? Thanks.
- Sun Nov 29, 2020 1:01 am
- Forum: Student Social/Study Group
- Topic: Week 8/9 Thoughts/Worries
- Replies: 66
- Views: 4635
Week 8/9 Thoughts/Worries
How does everyone feel about the class so far? I feel like it is going well, there are some points where I could've done better. Does anyone feel the same?
- Sun Nov 29, 2020 12:58 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: What is coplanar?
- Replies: 8
- Views: 5318
What is coplanar?
How does an even number lead to a coplanar on a molecule? Are coplanars important to molecule structure?
- Sun Nov 29, 2020 12:44 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3914643
Re: Post All Chemistry Jokes Here
Why are chemists so great at solving problems? Because they have all the solutions.
Why did the white bear dissolve in water? Because it was a polar bear.
A photon checks into a hotel and is asked if he needs any help with his luggage. He says, "No, I'm traveling light."
Why did the white bear dissolve in water? Because it was a polar bear.
A photon checks into a hotel and is asked if he needs any help with his luggage. He says, "No, I'm traveling light."
- Sun Nov 29, 2020 12:34 am
- Forum: Student Social/Study Group
- Topic: Chemistry Among Us
- Replies: 43
- Views: 2712
Re: Chemistry Among Us
Chapter 2: Yellow decides to follow Green. It was odd that Green would leave right as the reactor went off. Yellow thought to themselves, 'I better be careful, Green could be the Imposter.' You follow behind Green catching up with them in weapons. They're standing in front of the download data righ...
- Sun Nov 29, 2020 12:07 am
- Forum: Student Social/Study Group
- Topic: Studying From Home
- Replies: 91
- Views: 8974
Re: Studying From Home
I feel as though one way to make studying at home better is to: 1. Find an environment that suits you. 2. Mentally prepare and take care of yourself. 3. Plan out what you have to do, to keep yourself on track. 4. Write down anything you need clarification on, for any class. 5. Make time for office h...
- Sun Nov 29, 2020 12:03 am
- Forum: Sigma & Pi Bonds
- Topic: Delocalized π bonds
- Replies: 6
- Views: 273
Delocalized π bonds
So in terms of Lewis structures, delocalized π bonds can only occur with resonance structures, or even if a molecule doesn't have a resonance structure can delocalized π bonds exist? I'm referring to the solution of question 16 on Sapling just for clarification.
- Sun Nov 22, 2020 5:48 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Stable Geometry
- Replies: 2
- Views: 95
Stable Geometry
On one of the questions for Sapling, the solution mentioned that the most stable geometry is an arrangement that keeps the atoms or electrons bonded to the central atom as far apart as possible. Why is this true? Does it have something to do with electron-electron repulsion?
- Sun Nov 22, 2020 5:40 pm
- Forum: General Science Questions
- Topic: # of chem posts
- Replies: 27
- Views: 2009
Re: # of chem posts
As mentioned before you should have 35 posts and 50 by the end of the quarter. Make sure to check the dates to see how often you've been posting per week.
- Sun Nov 22, 2020 5:38 pm
- Forum: Student Social/Study Group
- Topic: Lewis Structure
- Replies: 4
- Views: 233
Re: Lewis Structure
For Lewis structures, the octet rule can be violated by elements that use the d-orbitals found in the third principal energy level and greater (n=3...5, 6). S, P, Si, and Cl are some examples of elements that form an expanded octet.
- Sun Nov 22, 2020 5:33 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 28290
Re: Exercising Our Minds and Bodies
Nane Onanyan 1H wrote:Learning the choreo for "I Cant Stop Me" by twice B)
That's amazing. Twice's choreos are always fun to learn. Hope you have fun.
- Sun Nov 22, 2020 5:31 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 28290
Re: Exercising Our Minds and Bodies
Recently, I've been really into doing yoga. I feel like it is really good to stretch out your body in the morning before sitting in front of a computer screen all day, lol.
- Sun Nov 22, 2020 5:29 pm
- Forum: General Science Questions
- Topic: Grades
- Replies: 30
- Views: 2376
Re: Grades
Like everyone else has mentioned, if you ever want to check your grades you can go to my.ucla.edu and go under the Classes tab to find grades and transcripts.
- Sat Nov 14, 2020 6:25 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Oxidation Numbers (Sapling Q.9)
- Replies: 2
- Views: 182
Oxidation Numbers (Sapling Q.9)
I know Sapling Question.9 asks, "What is the oxidation number of chlorine in the perchlorate ion?" How do I approach this question? Also, has Lavelle talked about oxidation numbers yet?
- Sat Nov 14, 2020 6:18 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2
- Replies: 22
- Views: 985
Re: Midterm 2
I've mostly been practicing using textbook problems and going through the Sapling explanations again. This has been the most helpful for me. Also, since this section is more conceptual try to explain the problems to yourself before you solve/approach them, that might be helpful.
- Sat Nov 14, 2020 6:12 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3914643
Re: Post All Chemistry Jokes Here
What was Avogadro’s favorite sport?
-Golf, because he always got a mole-in-one.
-Golf, because he always got a mole-in-one.
- Sat Nov 14, 2020 6:05 pm
- Forum: Bond Lengths & Energies
- Topic: Energy released
- Replies: 3
- Views: 205
Energy released
Which would release more energy, an intermolecular/interionic interaction or an intramolecular bond when broken?
- Sat Nov 14, 2020 5:50 pm
- Forum: Lewis Acids & Bases
- Topic: Acids and Bases Recognition
- Replies: 3
- Views: 303
Acids and Bases Recognition
I know for me I had trouble identifying Lewis acids and bases. Especially for Sapling problem # 6. I found this picture to be helpful as well as writing out the Lewis structures.
- Mon Nov 09, 2020 10:39 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2 Study Group
- Replies: 25
- Views: 1233
Re: Midterm 2 Study Group
This is so cool. Thanks for putting this together.
- Mon Nov 09, 2020 1:52 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Among Us
- Replies: 43
- Views: 2712
Re: Chemistry Among Us
Hi guys, I am back once more for some chemistry fun. This week I wanted to do Among Us, where I let you pick choices, and then the next day I respond with the next chapter/choice. Have fun. Chapter 1: The Missing Valence The clock reads 8:35 AM. You are Yellow, tasked with checking that all the val...
- Sun Nov 08, 2020 2:22 pm
- Forum: Student Social/Study Group
- Topic: Favorite TV shows
- Replies: 277
- Views: 51491
Re: Favorite TV shows
I 100% recommend watching the vampire diaries, gossip girl,new girl, young and hungry, and honestly I have so many more suggestions!! hmu if you need more ideas :) Your taste is amazing. I just finished binge-watching Vampire Diaries and The Originals too. Absolutely 10/10. I also started watching ...
- Sun Nov 08, 2020 2:19 pm
- Forum: Student Social/Study Group
- Topic: Favorite TV shows
- Replies: 277
- Views: 51491
Re: Favorite TV shows
Hello! First off, I've watched a LOT of the recommendations above + I would say all of them are really good! Second, I just wanted to add a recommendation of my own, especially if you like crime/forensics. It's called Bones, and I think it's on Hulu (?) Feel free to let me know what you think! Yes,...
- Sun Nov 08, 2020 2:08 pm
- Forum: Dipole Moments
- Topic: Hydrogen bonds
- Replies: 9
- Views: 629
Re: Hydrogen bonds
Like, everyone else has mentioned a hydrogen bond occurs when a highly electronegative N, O, or F atom shares a lone pair of electrons with an electron-deficient H atom. O---H---O for example, like #14 on Sapling.
- Sun Nov 08, 2020 1:47 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Among Us
- Replies: 43
- Views: 2712
Chemistry Among Us
Hi guys, I am back once more for some chemistry fun. This week I wanted to do Among Us, where I let you pick choices, and then the next day I respond with the next chapter/choice. Have fun. Chapter 2 is live scroll down to see it and leave your replies!!!! Chapter 3 is LIVEEEE! Scroll down and leave...
- Sun Nov 08, 2020 1:33 pm
- Forum: Student Social/Study Group
- Topic: How to relax
- Replies: 168
- Views: 36192
Re: How to relax
I love revisiting my childhood by watching all of my past favorite television shows. Furthermore, I find this so interesting as I am able to see the show from a different perspective than I did as a 6th grader in middle school. This allows me to understand parts of the show that I never was able to...
- Sun Nov 08, 2020 1:29 pm
- Forum: Resonance Structures
- Topic: Resonance & Stability
- Replies: 4
- Views: 251
Resonance & Stability
Why is a molecule with several resonance structures, more stable than one with fewer? Does it have something to do with the ability to change where the bonds are placed or the delocalization of electrons?
- Sun Nov 08, 2020 1:23 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Electronegativity & Formal Charge
- Replies: 3
- Views: 149
Electronegativity & Formal Charge
When calculating the formal charge, is it more favorable for any charges to be on the more electronegative atoms? Also, why do we not want our central atom to have a charge? Thanks.
- Sun Nov 08, 2020 1:14 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bonds
- Replies: 3
- Views: 176
Hydrogen Bonds
How does the size and shape of a molecule limit the number of hydrogen bonds formed? I was thinking about this from the Sapling question about the Urea molecule.
- Sat Oct 31, 2020 8:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework due date
- Replies: 49
- Views: 2593
Re: Homework due date
I would assume by Sunday night, they mean 11:59 pm Sunday. Especially, because it says "Sunday at Midnight."
- Sat Oct 31, 2020 8:32 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Wave Function Quantum Numbers
- Replies: 11
- Views: 360
Re: Wave Function Quantum Numbers
I also found a picture that assisted me. Hopefully, it can help a bit.
- Sat Oct 31, 2020 7:52 pm
- Forum: Student Social/Study Group
- Topic: Favorite TV shows
- Replies: 277
- Views: 51491
Favorite TV shows
Hey, everyone. I was wondering what are some good show recommendations to watch or some of your favorite childhood shows. Recently, I have watched Castlevania 10/10, and when I was younger I loved Total Drama Island, also a 10/10.
- Sat Oct 31, 2020 7:44 pm
- Forum: Photoelectric Effect
- Topic: Lyman Series
- Replies: 30
- Views: 1362
Re: Lyman Series
This is how I remembered it: The Lyman series corresponds to UV light and the electrons rest at the energy level n=1. While the Balmer series corresponds to visible light and the electron's rest at the energy level n=2.
- Sat Oct 31, 2020 7:40 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Atom and Ionic Radius
- Replies: 4
- Views: 273
Atom and Ionic Radius
How does electron repulsion contribute to the increase or decrease of the radius size? Thank you.
- Sat Oct 24, 2020 9:53 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Electron Transition Series
- Replies: 2
- Views: 221
Electron Transition Series
Are we going to be asked questions like “What series would this transition belong to?” On the midterm or is this something I shouldn’t worry about?
- Sat Oct 24, 2020 9:31 pm
- Forum: Student Social/Study Group
- Topic: How to relax
- Replies: 168
- Views: 36192
How to relax
What do you guys like to do to relax when not doing work? I usually watch Netflix, play with my dog, or play video games.
- Sat Oct 24, 2020 9:27 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg’s Constant
- Replies: 6
- Views: 360
Rydberg’s Constant
During one of the review sessions someone said something that peeked my interest. Is it true that N1 is initial if it’s absorption and final if it is emission?Because in the textbook it is written as 1/n1 first.
- Sat Oct 24, 2020 9:17 pm
- Forum: DeBroglie Equation
- Topic: Advice for Correct Units for Midterm
- Replies: 8
- Views: 406
Re: Advice for Correct Units for Midterm
Wow, I never really thought of this. Sometimes when solving the equations I just plug in the numbers and add the units at the end. This is very helpful, thank you.
- Sat Oct 24, 2020 9:12 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Multi-Electron Atoms
- Replies: 3
- Views: 179
Multi-Electron Atoms
What are the additional factors that effect the e- energy electrostatic potential energy in Multi-Electron Atoms? I know Lavelle mentioned it in his slides, but I don’t think I have a full grasp of the concept.


