Search found 108 matches
- Tue Mar 16, 2021 1:25 am
- Forum: Student Social/Study Group
- Topic: What do you miss / What are you looking forward to?
- Replies: 92
- Views: 10666
Re: What do you miss / What are you looking forward to?
I'm definitely looking forward to campus life and dorming.
- Tue Mar 16, 2021 1:22 am
- Forum: Student Social/Study Group
- Topic: Fave movie/show
- Replies: 67
- Views: 5097
Re: Fave movie/show
Grey's Anatomy has been a favorite for so long, Gilmore Girls, Friends, and Wandavision is a must at the moment
- Tue Mar 16, 2021 1:19 am
- Forum: Student Social/Study Group
- Topic: Points Required for Full Credit of Chem Community
- Replies: 11
- Views: 1048
Re: Points Required for Full Credit of Chem Community
As long as you have 50 in total, you're good!
- Tue Mar 16, 2021 1:14 am
- Forum: Student Social/Study Group
- Topic: Book Recommendations
- Replies: 135
- Views: 15983
Re: Book Recommendations
Anything written by Colleen Hoover is literal perfection
- Sat Mar 13, 2021 9:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: sign for Eo when reversing rxn
- Replies: 11
- Views: 818
Re: sign for Eo when reversing rxn
It depends! Be careful when you're multiplying by a factor because the sign stays the same.
- Sat Mar 13, 2021 9:50 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Oxidation and reduction
- Replies: 12
- Views: 1487
Re: Oxidation and reduction
Oxidation deals with the loss of electrons while reduction is when you gain electrons. It takes place in a Galvanic Cell when the left reaction is the oxidation reaction and the right reaction is the reduction reaction.
- Sat Mar 13, 2021 9:48 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: K = kforward/kreverse
- Replies: 16
- Views: 1171
Re: K = kforward/kreverse
This is correct, but make sure you acknowledge the units because k could also be used as the pseudo-rate constant in a reaction.
- Sat Mar 13, 2021 9:39 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Bonus Q's
- Replies: 7
- Views: 478
Re: Final Bonus Q's
I think they might be worth 4 points, as the bonus questions in the past midterms have been 4 points.
- Sat Mar 13, 2021 9:37 pm
- Forum: Student Social/Study Group
- Topic: Sapling good to review for final?
- Replies: 16
- Views: 972
Re: Sapling good to review for final?
Sapling is good for practice and for a step by step work down of the problems. I would say that textbook problems are a lot better for reviewing because the final is based off of the textbook problems.
- Sat Mar 13, 2021 9:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Daniell Cell
- Replies: 4
- Views: 380
Re: Daniell Cell
Because it wasn't mentioned in our lectures, I don't think you have to worry too much about it.
- Sat Mar 13, 2021 9:21 pm
- Forum: Student Social/Study Group
- Topic: Spring Quarter
- Replies: 60
- Views: 3587
Re: Spring Quarter
I'm going to be taking Chem 14C!
- Fri Mar 12, 2021 10:27 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Missed Chem Community Points
- Replies: 13
- Views: 1254
Re: Missed Chem Community Points
I think you'll be fine as long as you have 50 posts by the end of this course.
- Fri Mar 12, 2021 6:23 pm
- Forum: General Rate Laws
- Topic: Sapling 9&10 #7
- Replies: 10
- Views: 909
Re: Sapling 9&10 #7
Hi, for this problem, part 5 is not asking for a numerical answer, it is asking for the rate law expression. This means that we can use the orders for each substance found to write the rate law with the form rate=k[A] a [B] b [C] c , where a,b, and c are the orders for each component. Because we do...
- Fri Mar 12, 2021 6:22 pm
- Forum: General Rate Laws
- Topic: Sapling 9&10 #7
- Replies: 10
- Views: 909
Re: Sapling 9&10 #7
For part 5, since you know that [A] is first order, [B] is second order, and [C] is zero order, you can write the rate law as rate=k[A][B] 2 For part 6, pick one of the experiments and plug in the [A] and [B], as well as the initial rate, into the rate law above. That should help you solve for k. F...
- Fri Mar 12, 2021 6:22 pm
- Forum: General Rate Laws
- Topic: Sapling 9&10 #7
- Replies: 10
- Views: 909
Re: Sapling 9&10 #7
#Part 5: The generic rate law is: rate=k [A]^a [B]^b [C]^c k is the rate constant. [A], [B], and [C] are the initial concentrations of the reactants. a, b, and c are the orders of the reactants (A, B, and C). You found the order of the reactant A in part 1: 1st order --> a = 1 You found the order o...
- Fri Mar 12, 2021 10:57 am
- Forum: General Rate Laws
- Topic: Sapling 9&10 #7
- Replies: 10
- Views: 909
Sapling 9&10 #7
For the reaction 2A(g)+2B(g)+C(g)⟶3G(g)+4F(g) the initial rate data in the table was collected, where [A]0 , [B]0 , and [C]0 are the initial concentrations of A , B , and C , respectively. Screen Shot 2021-03-12 at 10.50.51 AM.png Part 1: Identify the order of reactant A. A: first-order reactant Par...
- Wed Mar 03, 2021 11:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Which Nesnst Equation
- Replies: 25
- Views: 1206
Re: Which Nesnst Equation
I believe it's better to use the ln equation, as it can be applied to any situation.
- Wed Mar 03, 2021 11:17 pm
- Forum: Student Social/Study Group
- Topic: What organizations are you guys in?
- Replies: 53
- Views: 3453
Re: What organizations are you guys in?
I'm a UniCamp volunteer!
- Sun Feb 28, 2021 11:08 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Thermodynamics delta G
- Replies: 13
- Views: 939
Re: Thermodynamics delta G
Gibbs acts as a state function and determines whether or not a reaction is spontaneous in a given reaction.
- Sun Feb 28, 2021 11:03 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling week 7/8 #18
- Replies: 25
- Views: 5082
Re: Sapling week 7/8 #18
Everything is right except for the 6H2O in product. It should be 3H2O
- Sun Feb 28, 2021 11:01 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #18
- Replies: 3
- Views: 212
Re: Sapling Week 7/8 #18
I had this problem too! Everything is correct, you just have to remove the parenthesis from the equation.
- Sun Feb 28, 2021 10:58 pm
- Forum: Student Social/Study Group
- Topic: Chem 14B Final
- Replies: 86
- Views: 6039
Re: Chem 14B Final
Textbook problems are a must! They help so much to review and absorb the content. Another way of studying is to watch specific focus topic videos on YouTube. Organic Chemistry Tutor is a great source and I rely on them a lot as well.
- Sun Feb 28, 2021 10:56 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling #4
- Replies: 6
- Views: 355
Re: Sapling #4
I don't think you reaction is correctly balanced. It should be 3HNO3, 3NO2, and 3H2O instead of the 4
- Sun Feb 28, 2021 10:50 pm
- Forum: Student Social/Study Group
- Topic: Final
- Replies: 63
- Views: 3761
Re: Final
Definitely recommend doing textbook problems! I focused a lot on completing and reviewing textbook problems for the midterm and was very satisfied with my grade.
- Sun Feb 28, 2021 10:46 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #5
- Replies: 9
- Views: 499
Re: Sapling Week 7/8 #5
For this problem I can't figure out how to balance the equation. I think I'm getting confused on what to do with the H20 and OH- in the beginning but I'm not sure. I got the oxidizing and reducing agents right but now I don't know what to do. Screen Shot 2021-03-01 at 1.27.01 AM.png You're on the r...
- Sun Feb 14, 2021 8:21 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Sapling #5
- Replies: 9
- Views: 521
Re: Sapling #5
I agree with Jaden. I find converting kPa to atms a lot simpler.
- Sun Feb 14, 2021 8:17 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2
- Replies: 3
- Views: 238
Re: Midterm 2
I'm assuming lectures 12+ since all of the content before lecture 12 were covered in Midterm 1.
- Sun Feb 14, 2021 7:32 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Sapling #6 weeks 5 & 6
- Replies: 2
- Views: 124
Sapling #6 weeks 5 & 6
A particular container holds 4.19 mol of neon gas. The volume of this container can be altered by sliding a piston in or out. The volume is changed from 8.00 L to 5.60 L while at the same time the temperature is changed from 275 K to 129 K. The molar heat capacity, CV,m, for neon is 12.47 J/(mol · K...
- Sun Feb 07, 2021 11:14 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling Week 3/4 #9
- Replies: 3
- Views: 168
Re: Sapling Week 3/4 #9
The side that should be negative is the hot water. It should be:
(mass of cold water)cΔT(cold water) = −(mass of hot water)cΔT(hot water)
I hope this helps!
(mass of cold water)cΔT(cold water) = −(mass of hot water)cΔT(hot water)
I hope this helps!
- Sun Feb 07, 2021 11:08 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm 1...
- Replies: 39
- Views: 1873
Re: Post Midterm 1...
I've been really reviewing textbook questions and watching videos on YouTube to better understand the content. Organic Chemistry Tutor is a great resource!
- Sat Feb 06, 2021 8:00 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: deltaU
- Replies: 29
- Views: 940
Re: deltaU
Delta U represents the change in internal energy. Sometimes delta U is symbolized as delta E instead, but they are essentially the same.
- Sat Feb 06, 2021 7:57 pm
- Forum: Student Social/Study Group
- Topic: Best kdrama?
- Replies: 30
- Views: 2053
Re: Best kdrama?
Scarlet Heart Ryeo had me crying all night
- Sat Feb 06, 2021 4:23 pm
- Forum: Calculating Work of Expansion
- Topic: Sapling WEEK 3/4 Q #20
- Replies: 8
- Views: 295
Re: Sapling WEEK 3/4 Q #20
The equation for Q = nC,vdeltaT
For your problem, n = 0.721 mol, deltaT = 14.5 K and R = 8.3145
So, just plug everything into the equation!
Q = 0.721 x (5(8.3145)/2) x (14.5) and in this case, Q = deltaU so whatever you get for Q is deltaU/internal energy.
Hope this helps!
For your problem, n = 0.721 mol, deltaT = 14.5 K and R = 8.3145
So, just plug everything into the equation!
Q = 0.721 x (5(8.3145)/2) x (14.5) and in this case, Q = deltaU so whatever you get for Q is deltaU/internal energy.
Hope this helps!
- Sat Feb 06, 2021 4:14 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Sapling question
- Replies: 3
- Views: 223
Re: Sapling question
If you're referring to #9 week 3 & 4, the question is asking you to solve for Tfinal. To do that, you would need to figure out the heat gained by cold water, which is: Heat gained by cold water = (Mass)(Specific heat capacity of water)(Change in temperature) --> plug in your given values! Then f...
- Sat Feb 06, 2021 1:11 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Sapling Week 5/6 #5
- Replies: 1
- Views: 120
Re: Sapling Week 5/6 #5
Since you've already calculated the number of moles, you would then solve for delta S using the equation: n x Cv,m x ln ( Tfinal -Tinitial). Plug all of your given and solved values in and you should get the answer! I hope this helps :)
- Sat Feb 06, 2021 12:03 pm
- Forum: Student Social/Study Group
- Topic: TEST BANKS?
- Replies: 6
- Views: 916
Re: TEST BANKS?
Looks like there's one pretty old one from Lavelle on here, but nothing super recent https://www.bruintestbank.com/chemistry/ On pg. 7 of the posted Midterm, it says "everything on this page came from this quarter's Chemistry Community" ... do you think or know whether Professor will be u...
- Sat Feb 06, 2021 11:53 am
- Forum: Student Social/Study Group
- Topic: Sapling HW and exams
- Replies: 19
- Views: 850
Re: Sapling HW and exams
I personally think Sapling is good practice for problem solving. I like how it shows you the step by step on how to solve the problems after.
- Fri Feb 05, 2021 11:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling Week 3 & 4 #11
- Replies: 4
- Views: 246
Re: Sapling Week 3 & 4 #11
The heat lost or absorbed by a substance is given by the equation: q=mcΔT. Since the heat the water takes in is equal to the heat that leaves the aluminum, qwater=−qmetal. From this, you can use q=mcΔT for the water and then for the metal, setting up the equation mcΔT(water) = -mcΔT(metal) Because ...
- Fri Feb 05, 2021 11:03 pm
- Forum: Student Social/Study Group
- Topic: Midterm Review
- Replies: 7
- Views: 913
Re: Midterm Review
Textbook problems and watching Organic Chemistry Tutor videos on YouTube always helps me!
- Thu Feb 04, 2021 10:41 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling Week 3 & 4 #11
- Replies: 4
- Views: 246
Sapling Week 3 & 4 #11
A hot lump of 49.9 g of copper at an initial temperature of 79.0 °C is placed in 50.0 mL H2O initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the copper and water, given that the specific heat of copper is 0.385 J/(g·°C)? Assume no heat is lost to surro...
- Thu Feb 04, 2021 10:21 pm
- Forum: Phase Changes & Related Calculations
- Topic: Sapling week 3 and 4 #10
- Replies: 3
- Views: 228
Re: Sapling week 3 and 4 #10
First, make sure you convert mass of ice to moles of ice. Once you've done that conversion, multiply that number by the standard enthalpy of fusion for water. Then, calculate the heat flow (q) because we need to know the heat required to raise the temperature of the melted ice to the final temperatu...
- Thu Feb 04, 2021 10:06 pm
- Forum: Calculating Work of Expansion
- Topic: Sapling week 3/4 #15
- Replies: 9
- Views: 561
Re: Sapling week 3/4 #15
Start by converting mass to moles. Once you've done that, use the ideal gas law, PV=nRT to solve for V. From there, use w=−pV to solve for w.
- Thu Feb 04, 2021 10:01 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Constant pressure/ volume
- Replies: 11
- Views: 492
Re: Constant pressure/ volume
When we refer to constant pressure, we are saying that the only thing that stays constant is the pressure. Anything else can change. This is the same with constant volume.
- Thu Feb 04, 2021 9:59 pm
- Forum: Ideal Gases
- Topic: Chem BL
- Replies: 107
- Views: 8901
Re: Chem BL
I'll either take 14C and then BL or take them both at the same time. I haven't decided yet if I want to take it next quarter, but it seems like a good idea to do.
- Mon Feb 01, 2021 9:45 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 4
- Views: 300
Re: Midterm
Last quarter, exam grades usually went out a week after. I'm assuming it'll be the same this quarter!
- Fri Jan 29, 2021 11:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy solving methods
- Replies: 7
- Views: 355
Enthalpy solving methods
When solving for enthalpy can we use any of the three methods Dr. Lavelle taught us or is there a recommended method we should use for certain problems? Is there a specific one you prefer using?
- Sun Jan 17, 2021 10:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Week 1 Sapling #10
- Replies: 6
- Views: 216
Re: Week 1 Sapling #10
I also can't figure out the second part of this problem! I can't seem to figure out what I'm doing wrong.
- Sun Jan 17, 2021 10:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling #10
- Replies: 4
- Views: 325
Sapling #10
I'm having a hard time with the last part of this problem: Calculate the equilibrium concentrations of N2O4 and NO2 after the extra 1.00 mol NO2 is added to 1.00 L of solution. [N2O4]= [NO2]= The Kc that I calculated is 11.2. I think my problem is solving for x. I set the equation up: [NO2]^2 / [N2O...
- Sun Jan 17, 2021 6:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling #4
- Replies: 6
- Views: 236
Sapling #4
Hi! I'm having trouble solving this problem and keep getting the wrong answer. At a certain temperature, the given reaction has an equilibrium constant of Kp=359 . PCl3(g)+Cl2(g)↽−−⇀PCl5(g) PCl5 is placed in a sealed container at an initial pressure of 0.0370 bar . What is the total pressure at equi...
- Sat Jan 16, 2021 11:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling HW #2
- Replies: 6
- Views: 329
Re: Sapling HW #2
Mitzy Garcia 1k wrote:Yes, use the ICE table. I converted the units for SO3 and O2. I did this by using mol and L to equal to M. I hope that helps.
This is very helpful!! Thank you (:
- Sat Jan 16, 2021 9:36 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Using the ICE table
- Replies: 36
- Views: 1470
Re: Using the ICE table
I believe you would be able to use the ICE table for both concentration and partial pressures! This is a great tool to keep everything organized.
- Sat Jan 16, 2021 9:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling HW #2
- Replies: 6
- Views: 329
Sapling HW #2
At a certain temperature, 0.860 mol SO3 is placed in a 4.00 L container.
2SO3(g)↽−−⇀2SO2(g)+O2(g)
At equilibrium, 0.180 mol O2 is present. Calculate Kc.
I'm having a little bit of trouble for this question. Would I just start by creating an ICE table?
2SO3(g)↽−−⇀2SO2(g)+O2(g)
At equilibrium, 0.180 mol O2 is present. Calculate Kc.
I'm having a little bit of trouble for this question. Would I just start by creating an ICE table?
- Sat Jan 16, 2021 9:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Calculator for exams
- Replies: 28
- Views: 1013
Re: Calculator for exams
Last quarter, my TA said we were fine using any calculator, so I'm assuming it's the same this quarter.
- Wed Jan 13, 2021 9:16 pm
- Forum: Administrative Questions and Class Announcements
- Topic: What units will be in Midterm 1?
- Replies: 6
- Views: 297
Re: What units will be in Midterm 1?
I believe it should be Units 1, 2 and maybe some of 3.
- Tue Jan 05, 2021 9:01 pm
- Forum: Student Social/Study Group
- Topic: Learning Sessions
- Replies: 24
- Views: 1223
Re: Learning Sessions
It really depends on what you prefer! Last quarter I attended more sessions before/during midterms or finals week to prepare. That helped a lot for review and practice.
- Wed Dec 16, 2020 2:29 pm
- Forum: Student Social/Study Group
- Topic: Changing Study Habits
- Replies: 35
- Views: 1508
Re: Changing Study Habits
Attending the UA sessions were really helpful! I would say going to as many of those as you can and also completing textbook problems because the exam questions are very similar to the homework. Also! I found watching videos from Organic Chemistry Tutor really helpful as well! Hope this helps.
- Wed Dec 16, 2020 2:25 pm
- Forum: Lewis Acids & Bases
- Topic: Acids & Bases
- Replies: 9
- Views: 814
Re: Acids & Bases
For me, I go off the name of the compound. Acids usually have a H at the beginning of the name, so I typically identify the acids first and then go off from there.
- Wed Dec 16, 2020 12:07 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: What are the bond angles for T shaped?
- Replies: 21
- Views: 8760
Re: What are the bond angles for T shaped?
Bond angles for T shapes are a little less than or equal to 90 degrees!
- Wed Dec 16, 2020 11:54 am
- Forum: *Titrations & Titration Calculations
- Topic: Is this a 14B topic? [ENDORSED]
- Replies: 8
- Views: 1084
Re: Is this a 14B topic? [ENDORSED]
In Dr.Lavelle's lectures, I remember him mentioning that we'll be going further into acids and bases in Chem 14B.
- Wed Dec 16, 2020 11:52 am
- Forum: Student Social/Study Group
- Topic: Grades
- Replies: 29
- Views: 1372
Re: Grades
Grades should be out Saturday! And I believe that you have until 10am Thursday now to get 50 chem community posts.
- Fri Dec 11, 2020 8:53 pm
- Forum: Student Social/Study Group
- Topic: Studying
- Replies: 13
- Views: 949
Re: Studying
I've been referring to Organic Chemistry Tutor and they've really helped me improve a lot!!
- Fri Dec 11, 2020 8:49 pm
- Forum: Student Social/Study Group
- Topic: Struggling on topics
- Replies: 6
- Views: 439
Re: Struggling on topics
If there's a topic I'm struggling on, I like to do practice problems (or hw problems) until I'm confident that I know what I'm doing. Organic Chemistry Tutor on YouTube is also a great resource!
- Fri Dec 11, 2020 8:46 pm
- Forum: General Science Questions
- Topic: What time is the final?
- Replies: 5
- Views: 730
Re: What time is the final?
If you're international, there's also a time for 8pm - 9:30pm.
- Sun Dec 06, 2020 1:19 am
- Forum: Student Social/Study Group
- Topic: Studying for Final Exam
- Replies: 57
- Views: 2904
Re: Studying for Final Exam
I'm going to be going through the textbook problems and also attending the UA zoom sessions, as doing that has worked best so far in improving my grade.
- Wed Dec 02, 2020 10:05 pm
- Forum: General Science Questions
- Topic: Grade Breakdown
- Replies: 35
- Views: 2433
Re: Grade Breakdown
I'm honestly curious about this as well! As far as I know, and like everyone else has said, 50% is needed to pass with a C. I think the most important thing is to get enough points as you can.
- Sun Nov 29, 2020 10:38 pm
- Forum: Ionic & Covalent Bonds
- Topic: Lewis structure
- Replies: 13
- Views: 728
Re: Lewis structure
Hi! What has worked for me is referring to my periodic table. I know that elements that are closer to each other on the periodic table are ionic and elements further away are covalent. Hope this helps!
- Sun Nov 29, 2020 10:36 pm
- Forum: Sigma & Pi Bonds
- Topic: sapling #15
- Replies: 24
- Views: 1215
Re: sapling #15
I determine how many sigma and pi bonds are in a molecule based on the structure! There is one sigma bond present in every double bond and single bond while pi bonds are found in double and triple bonds. For instance, if there was a triple bond, there would be one sigma bond and two pi bonds. I hope...
- Sun Nov 29, 2020 10:33 pm
- Forum: Student Social/Study Group
- Topic: studying for exams
- Replies: 21
- Views: 2537
Re: studying for exams
For me, I review sapling and also do the textbook problems as practice. Because Dr. Lavelle takes textbook problems for the test, I find this really helpful since the questions are similar. This helped me do a lot better on the second midterm.
- Fri Nov 27, 2020 11:13 pm
- Forum: Student Social/Study Group
- Topic: Sampling Give Up Option
- Replies: 12
- Views: 599
Re: Sampling Give Up Option
Sapling is set up so that if you give up on a question, it makes you lose points. Try doing as many attempts as you can because you get an unlimited number of attempts!
- Fri Nov 27, 2020 10:57 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Outlines for the Final
- Replies: 11
- Views: 681
Re: Outlines for the Final
I believe they will be covered before the final since we'll be examined on all of the material.
- Sun Nov 22, 2020 6:52 pm
- Forum: Student Social/Study Group
- Topic: Big Sad: Midterm 2
- Replies: 86
- Views: 6866
Re: Big Sad: Midterm 2
I felt the same way the first midterm so I totally understand. Just remember that there are a lot of points you can earn in addition to the midterm! Rooting for you (:
- Sun Nov 22, 2020 6:51 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2
- Replies: 9
- Views: 575
Re: Midterm 2
I'm assuming they will come out later this week. (:
- Wed Nov 18, 2020 11:21 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Power vs Polarizability
- Replies: 4
- Views: 348
Polarizing Power vs Polarizability
Hello! I was completing the text book problems and got a bit confused when asked to arrange cations from increasing polarizing power and anions from increasing polarizability. I mixed the two up at first. What is the difference between polarizing power and polarizability?
Thank you!
Thank you!
- Tue Nov 17, 2020 2:02 pm
- Forum: Student Social/Study Group
- Topic: Midterm Studying
- Replies: 3
- Views: 255
Midterm Studying
In preparation for the midterm, should we be focusing on textbook problems 1D, 1E, 1F and all of outline 3 textbook problems?
- Mon Nov 16, 2020 10:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Textbook Problem 1D.23
- Replies: 3
- Views: 310
Re: Textbook Problem 1D.23
Hi! It's true that the 4d subshell has 5 orbitals (-2, -1, 0, +1, +2). However, this information only fits with n = 4 and l = 2. The question adds on that the magnetic quantum number must be -2. This refers to only one of the 5 orbitals (the one marked -2), so the answer is one orbital (only one or...
- Mon Nov 16, 2020 10:05 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2
- Replies: 31
- Views: 1405
Re: Midterm 2
Jasmine Yi 1H wrote:cady_tran_2G wrote:I believe so! I might be wrong but I think Midterm 2 should cover the last of outline 2 and some of outline 3.
does this mean that Midterm2 doesn't cover all of outline 3?
It covers up to the lecture we had on 11/11.
- Mon Nov 16, 2020 10:00 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Textbook Problem 1D.23
- Replies: 3
- Views: 310
Textbook Problem 1D.23
How many orbitals can have the following quantum numbers in an atom: (b) n=4, l=2, 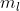 = -2
= -2
Hi! I'm studying for the midterm and I'm confused as to why when:
n = 4,
l = 2,
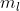 = -2
= -2
The number of orbitals is 1. Shouldn't it be 5 orbitals because the atom is at the 4d state?
Hi! I'm studying for the midterm and I'm confused as to why when:
n = 4,
l = 2,
The number of orbitals is 1. Shouldn't it be 5 orbitals because the atom is at the 4d state?
- Sat Nov 14, 2020 10:54 pm
- Forum: Student Social/Study Group
- Topic: grade worries
- Replies: 119
- Views: 21044
Re: grade worries
I have the same concerns as you did since I didn't do as well as I would have hoped for the first midterm. Hopefully the next midterm and final makes up for it!
- Sat Nov 14, 2020 7:13 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 3d Exceptions
- Replies: 3
- Views: 301
Re: 3d Exceptions
I agree with Audrey. This is a really good video that helped me understand this electron configuration exceptions: https://youtu.be/Ur2PuzJ7KWs
Hope this helps!
Hope this helps!
- Sat Nov 14, 2020 7:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 2: Lectures
- Replies: 5
- Views: 331
Re: Midterm 2: Lectures
I believe that Wednesday was the last lecture that covers material on the midterm. Just note that for midterm 2, it's going to cover the last 8 points of outline 2 and all of outline 3!
- Thu Nov 12, 2020 9:38 pm
- Forum: Ionic & Covalent Bonds
- Topic: Midterm 2
- Replies: 6
- Views: 386
Re: Midterm 2
Midterm 2 should cover the last 8 points on outline 2 and everything in outline 3.
- Mon Nov 09, 2020 11:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chem 14B and Chem 14BL
- Replies: 10
- Views: 631
Re: Chem 14B and Chem 14BL
I'm also considering taking 14B and 14BL together but it honestly depends on what you prefer. I feel like it would be a good to take them during winter quarter, but you also have to consider how many classes you plan to take overall and if this is going to significantly affect your workload. I hope ...
- Fri Nov 06, 2020 7:46 pm
- Forum: Student Social/Study Group
- Topic: Blind sided by Midterm 1 memorization questions, How to study for memorization questions
- Replies: 11
- Views: 398
Re: Blind sided by Midterm 1 memorization questions, How to study for memorization questions
Like everyone said previously, writing things down helps me memorize things but also going through Quizlet flashcards really helps as well!
- Fri Nov 06, 2020 7:43 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework due date
- Replies: 49
- Views: 2371
Re: Homework due date
This week's homework is due on Sunday of week 6. (:
- Tue Nov 03, 2020 7:42 pm
- Forum: Lewis Structures
- Topic: Octet/Double Bonds when drawing Lewis Structure
- Replies: 3
- Views: 198
Octet/Double Bonds when drawing Lewis Structure
Hello! I might have missed this in the lecture, but when you're drawing a Lewis Structure, how do you identify whether or not an element is octet?
- Mon Nov 02, 2020 1:47 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Fundamentals for Midterm 2
- Replies: 5
- Views: 216
Re: Fundamentals for Midterm 2
Dr. Lavelle hasn't specified yet, but I'm assuming the last bullets of outline 2 and some of outline 3. Hope this helps!
- Mon Nov 02, 2020 1:44 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2
- Replies: 31
- Views: 1405
Re: Midterm 2
I believe so! I might be wrong but I think Midterm 2 should cover the last of outline 2 and some of outline 3.
- Fri Oct 30, 2020 11:51 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Access Code
- Replies: 5
- Views: 329
Re: Sapling Access Code
I know a few people have had this problem and they've all emailed the UCLA store, as Tianjian suggested. I think that would be the best solution to get your code ASAP.
- Fri Oct 30, 2020 11:47 pm
- Forum: Properties of Light
- Topic: How to remember what v is in equations
- Replies: 46
- Views: 7453
Re: How to remember what v is in equations
When I refer to velocity, I know that it is the standard, "v" but when I'm doing an equation that involves frequency, I know that the font for "v" is different.
- Fri Oct 30, 2020 11:46 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Exam Scores
- Replies: 15
- Views: 758
Re: Midterm Exam Scores
I'm also unsure! But as everyone has said, there was an error so I'm sure the TA's will have them all reviewed by the upcoming week or so. Hopefully soon!
- Wed Oct 28, 2020 8:07 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect post module
- Replies: 4
- Views: 230
Photoelectric Effect post module
Hi! I'm a bit lost on how to solve problem 31) on the post assessment. The problem is:
A. If 3.607 x 10-19 J is required to remove an electron with zero kinetic energy from a metal surface, what would be the longest wavelength light that could do this?
A. If 3.607 x 10-19 J is required to remove an electron with zero kinetic energy from a metal surface, what would be the longest wavelength light that could do this?
- Mon Oct 26, 2020 11:04 pm
- Forum: DeBroglie Equation
- Topic: De Broglie wavelength
- Replies: 3
- Views: 137
Re: De Broglie wavelength
Hi! I agree with Justin. From the previous lectures, Dr. Lavelle mentioned that anything after 10^-15 would be observable. Anything under that would not be. Hope this helps!
- Fri Oct 23, 2020 10:30 pm
- Forum: General Science Questions
- Topic: Practice problems for midterm
- Replies: 3
- Views: 257
Re: Practice problems for midterm
My TA said that the midterm is probably going to be similar to the post module assessments and that doing the assessments up to quantum world would be a great tool for studying. Hope this helps!
- Fri Oct 23, 2020 10:27 pm
- Forum: Properties of Electrons
- Topic: Rydberg
- Replies: 10
- Views: 496
Re: Rydberg
I think nf and ni is the same as n1 and n2, but to be safe, I would also stick to using n1 and n2.
- Thu Oct 22, 2020 8:47 am
- Forum: Einstein Equation
- Topic: Sapling
- Replies: 2
- Views: 174
Sapling
Hi! The question is: When a metal was exposed to photons at a frequency of 1.00×1015 s−1, electrons were emitted with a maximum kinetic energy of 3.90×10^−19 J. Calculate the work function, Φ, of this metal. What is the maximum number of electrons that could be ejected from this metal by a burst of ...
- Wed Oct 21, 2020 11:31 pm
- Forum: Properties of Electrons
- Topic: intensity vs energy
- Replies: 29
- Views: 3393
Re: intensity vs energy
Like everyone said, this is false. I was confused about this too, but from what I gathered, a higher intensity doesn't increase the energy, it just increases the number of photons. The photoelectric effects states that if the energy of the photon is greater or equal to the energy to remove an electr...
- Mon Oct 19, 2020 7:38 pm
- Forum: SI Units, Unit Conversions
- Topic: SI units
- Replies: 9
- Views: 266
Re: SI units
Hi! You should use meters because when you calculate the equations, the units will cancel out so you're left with meters. If the question asks you for the answer in nm, you would then convert m to nm.
- Fri Oct 16, 2020 4:59 pm
- Forum: Einstein Equation
- Topic: m vs nm
- Replies: 66
- Views: 3784
Re: m vs nm
It definitely depends on the question, but if it doesn't specifically state what units to use, I think it would be okay to stick with meters.
- Thu Oct 15, 2020 7:40 am
- Forum: Photoelectric Effect
- Topic: Frequency vs. Intensity
- Replies: 16
- Views: 720
Frequency vs. Intensity
Hi! In the lecture, Dr. Lavelle mentioned that high intensity light would not emit electrons, but higher frequency light would. What's the difference between frequency and intensity?
- Wed Oct 14, 2020 8:08 pm
- Forum: Photoelectric Effect
- Topic: Electron not emitted even for high intensity light
- Replies: 9
- Views: 384
Re: Electron not emitted even for high intensity light
Hi! I'm not sure if this will be much help, but in my notes I wrote that even though there is high intensity light, the energy of the light has to be equal to or larger than the energy to remove in order for the electrons to be emitted. Also, if the intensity increases, the number of photons double....

