Search found 101 matches
- Fri Mar 12, 2021 5:06 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: R constant
- Replies: 6
- Views: 511
Re: R constant
Typically 8.314JK^-1mol^-1 is used, but it really depends on the units of other variables that are given.
- Fri Mar 12, 2021 5:00 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Zero Order rate law
- Replies: 2
- Views: 178
Re: Zero Order rate law
For a zero order reaction, change in concentration=-k*t. K is independent of any factors.
- Fri Mar 12, 2021 4:55 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalyst
- Replies: 30
- Views: 2174
Re: Catalyst
Catalyst will not appear in the overall equation since it is consumed then produced later. It has no effect on the overall reaction except increasing the rate of reaction.
- Fri Mar 12, 2021 4:53 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate Laws for Slow Step/Overall
- Replies: 14
- Views: 949
Re: Rate Laws for Slow Step/Overall
Intermediates are not included in the overall rate law because it is produced and later consumed. If it is present in the slowest step, then you need to use some relationship you get in other equations to substitute for.
- Fri Mar 12, 2021 4:46 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: K value
- Replies: 20
- Views: 864
Re: K value
K is a constant, therefore it is considered as positive value. It is only the number that matters.
- Sat Mar 06, 2021 7:16 pm
- Forum: General Rate Laws
- Topic: Factors Affecting k
- Replies: 83
- Views: 5708
Re: Factors Affecting k
The rate constant changes as temperature changes. Increasing the temperature increases rate constant K.
- Sat Mar 06, 2021 7:13 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation Energy and Energy of a Reaction
- Replies: 10
- Views: 626
Re: Activation Energy and Energy of a Reaction
Increasing the temperature of a reaction increases the rate of the reaction. This is because at higher temperature, molecules have higher kinetic energy hence collide with each other more often, resulting in more products formed per unit time.
- Sat Mar 06, 2021 7:10 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation Energy
- Replies: 17
- Views: 992
Re: Activation Energy
They are NOT the same thing. Activation energy is the energy barrier you need to overcome for any reaction to happen. Change in enthalpy is the energy gained/released in a reaction.
- Sat Mar 06, 2021 7:08 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Instantaneous Rate
- Replies: 41
- Views: 2288
Re: Instantaneous Rate
The instantaneous rate is high at the beginning of a reaction because there are many reactants available. As the reaction proceeds, reactants are gradually depleted, so the instantaneous rate decreases. When the reaction ends/reaches equilibrium, the instantaneous rate will become 0.
- Sat Mar 06, 2021 7:03 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Reaction/Average Rate
- Replies: 13
- Views: 771
Re: Reaction/Average Rate
Average rate is the overall rate of a reaction. It is calculated simply by change in concentration/change in time taken. However, rate of a reaction refers to the instantaneous rate at a particular time. It is numerically equal to the slope of the tangent line at that point.
- Sat Feb 27, 2021 7:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: anode / Cathode
- Replies: 9
- Views: 631
Re: anode / Cathode
It depends on the types of the cell. But typically, anode is the side where oxidation reaction happens("an ox"). Cathode is the side where reduction reaction happens.
- Sat Feb 27, 2021 7:52 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation vs Reduction
- Replies: 30
- Views: 1398
Re: Oxidation vs Reduction
Oxidation: loss of electrons/increase in oxidation number
Reduction: gain of electrons/decrease in oxidation number
Reduction: gain of electrons/decrease in oxidation number
- Sat Feb 27, 2021 7:48 pm
- Forum: Balancing Redox Reactions
- Topic: Priority in assigning oxidation numbers
- Replies: 6
- Views: 436
Re: Priority in assigning oxidation numbers
Typically, metals have positive oxidation number while non-metals have negative oxidation number. This is due to the fact that metals have a tendency to lose electrons while non-metals have a tendency to gain electrons.
- Sat Feb 27, 2021 7:46 pm
- Forum: Balancing Redox Reactions
- Topic: Determining which molecule is the oxidizing agent
- Replies: 49
- Views: 1999
Re: Determining which molecule is the oxidizing agent
Yes, the oxidizing agent is the one that gets reduced in a chemical equation(or experiencing a drop in oxidation number).
- Sat Feb 27, 2021 7:45 pm
- Forum: Balancing Redox Reactions
- Topic: Determining which molecule is the oxidizing agent
- Replies: 49
- Views: 1999
Re: Determining which molecule is the oxidizing agent
Yes, the oxidizing agent is the one that gets reduced in a chemical equation(or experiencing a drop in oxidation number).
- Sat Feb 27, 2021 7:44 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Acidic Versus Basic Solutions
- Replies: 20
- Views: 939
Re: Balancing Acidic Versus Basic Solutions
In acidic solution we use H+ while in basic solution we use OH- instead.
- Sat Feb 20, 2021 8:45 pm
- Forum: Balancing Redox Reactions
- Topic: Cell/battery
- Replies: 26
- Views: 1005
Re: Cell/battery
I think a cell and a battery is the same thing.
- Sat Feb 20, 2021 8:44 pm
- Forum: Balancing Redox Reactions
- Topic: Pt in Cell Diagram
- Replies: 14
- Views: 949
Re: Pt in Cell Diagram
Pt is used when we are using hydrogen as our electrode or an aqueous solution as our electrode, such as Fe3+, Fe2+|Pt. Pt is used when no other metal is available.
- Sat Feb 20, 2021 8:36 pm
- Forum: Balancing Redox Reactions
- Topic: Chemical Reactions and Electrical Energy
- Replies: 6
- Views: 351
Re: Chemical Reactions and Electrical Energy
Chemical reaction doesn't produce electrical energy. Chemical energy generated by a chemical reaction is converted into electrical energy.
- Sat Feb 20, 2021 8:35 pm
- Forum: Balancing Redox Reactions
- Topic: Salt Bridges
- Replies: 21
- Views: 866
Re: Salt Bridges
We introduce salt bridge to balance the charges since cations are released in anode and electrons are gained in cathode, otherwise the whole system will be charged.
- Sat Feb 20, 2021 8:34 pm
- Forum: Balancing Redox Reactions
- Topic: First Lecture Galvanic Cell
- Replies: 7
- Views: 465
Re: First Lecture Galvanic Cell
For Zn and Cu, we would use solutions that contains Zn2+ and Cu2+ ions such as ZnSO4 and CuSO4.
- Sat Feb 13, 2021 8:08 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Low temp making DeltaG negative?
- Replies: 8
- Views: 436
Re: Low temp making DeltaG negative?
Think about the equation deltaG0=deltaH-T*deltaS. A low temperature would still make the reaction not spontaneous if deltaH is positive and deltaS is negative. DeltaG0 is still positive.
- Sat Feb 13, 2021 8:04 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Examples of G, W, and U
- Replies: 3
- Views: 241
Re: Examples of G, W, and U
Q is the heat energy supplied/lost by a system. For example, when you heat up a kettle of water, the water is gaining heat energy. W is the work done on/by the system. For example, when you pump gas into your bicycle tyre, you are compressing the gas tool so work is done on the gas inside the gas to...
- Sat Feb 13, 2021 7:58 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard Gibbs Free Energy
- Replies: 8
- Views: 512
Re: Standard Gibbs Free Energy
What is the difference between gibbs free energy and standard gibbs free energy? As its name suggests, standard Gibbs free energy is the standard value for a reaction. However, in reality, a lot of reaction aren't happening under standard conditions, so that's why we are using Gibbs free energy(del...
- Sat Feb 13, 2021 7:53 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Units
- Replies: 20
- Views: 763
Re: Units
It all depends on the question, I would say both KJ and J are ok.
- Sat Feb 13, 2021 7:50 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Relationship between K and deltaGº
- Replies: 4
- Views: 263
Re: Relationship between K and deltaGº
deltaG0= -RT*lnK. According to the equation, when K<1, deltaG0 is positive(meaning the reaction is not spontaneous, reactants are favored); when K>1, deltaG0 is negative(meaning the reaction is spontaneous, products are favored).
- Sat Feb 06, 2021 7:34 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Enthalpy vs Entropy
- Replies: 7
- Views: 263
Re: Enthalpy vs Entropy
Enthalpy: the amount of heat energy gained/lost by a system.
Entropy: a measure of the degree of disorderness of a system.
Entropy: a measure of the degree of disorderness of a system.
- Sat Feb 06, 2021 7:33 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: How to know the sign for work
- Replies: 26
- Views: 1042
Re: How to know the sign for work
Temperature increases could also due to heat energy being supplied to the system. With regard to work done, work done is positive when the system gains energy(ex. compressing a gas) and is negative then the system loses energy(ex. when gas expands).
- Sat Feb 06, 2021 7:20 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Reversible vs. Irreversible
- Replies: 8
- Views: 345
Re: Reversible vs. Irreversible
All reactions are reversible to some degree. I believe you are talking about whether an EXPANSION is reversible. In this case, expansion is reversible if pressure of system=pressure of surrounding.
- Sat Feb 06, 2021 7:17 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Postive vs. negative work
- Replies: 18
- Views: 1052
Re: Postive vs. negative work
Basically, when you compress something, work done is positive. When something expands, the work done is negative. The reason being that when you do work to the system(ex. compressing a gas), the system gains energy so work done is positive. When the system itself does work(ex. when gas expands), the...
- Sat Feb 06, 2021 7:13 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: delta U= delta H
- Replies: 21
- Views: 1607
Re: delta U= delta H
When there is no work done. In other words, delta U=delta H when there is no change in volume, ex. in an ideal gas.
- Fri Jan 29, 2021 7:51 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: q vs delta H
- Replies: 5
- Views: 314
Re: q vs delta H
q is the energy supplied to the system while H is the enthalpy of a reaction. Depending on the situation, sometimes they are numerically the same thing.
- Fri Jan 29, 2021 7:30 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: change in celsius = change in kelvin
- Replies: 22
- Views: 8014
Re: change in celsius = change in kelvin
Kelvin=Celsius+273.15. So change in celsius = change in kelvin, it is only a matter of different units. For example, 298k-273k=25k=25c-0c=25c.
- Fri Jan 29, 2021 7:18 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: mass in heat calculation
- Replies: 3
- Views: 163
Re: mass in heat calculation
If the substance dissolve in water, then it is WATER your are heating up! So you will put 100g as the mass.
- Fri Jan 29, 2021 7:16 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: how to tell when ΔU is negative
- Replies: 4
- Views: 264
Re: how to tell when ΔU is negative
Raising the temperature means heat energy is supplied to the system so q is positive. Note that the questions assumes ideal gas behavior, so there is no change in W. As a result, there is a positive change in U.
- Fri Jan 29, 2021 7:07 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Kelvin or Celsius?
- Replies: 86
- Views: 5905
Re: Kelvin or Celsius?
Temperature is in Kelvin!
- Fri Jan 22, 2021 8:29 pm
- Forum: Phase Changes & Related Calculations
- Topic: Exothermic example
- Replies: 9
- Views: 631
Re: Exothermic example
Burning gasoline releases heat so it is an exothermic reaction!
- Fri Jan 22, 2021 8:27 pm
- Forum: Phase Changes & Related Calculations
- Topic: Standard enthalpy of formation
- Replies: 4
- Views: 262
Re: Standard enthalpy of formation
The standard enthalpy of formation for N2 and O2 is 0! Because they are in their naturally occurring state! That is why we use Hess's Law to calculate the delta H.
- Fri Jan 22, 2021 8:21 pm
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic v. Exothermic
- Replies: 139
- Views: 15489
Re: Endothermic v. Exothermic
I think at least according to the definition, endothermic reaction has positive H while exothermic reaction has negative H.
- Fri Jan 22, 2021 8:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: H and q
- Replies: 47
- Views: 1786
Re: H and q
I think q typically denotes a change in heat energy while H denotes a change in enthalpy.
- Fri Jan 22, 2021 8:15 pm
- Forum: Phase Changes & Related Calculations
- Topic: Vapor vs gas
- Replies: 121
- Views: 12638
Re: Vapor vs gas
I think for the sake of this course, vapor and gas are the same thing. However, at least for me, I think gas typically refers to something that is naturally in gas phase, such as nitrogen and hydrogen, while vapor refers to something that is "vaporized" into gas, such as water vapor and io...
- Fri Jan 15, 2021 11:35 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature
- Replies: 45
- Views: 1479
Re: Temperature
We need to first determine whether the forward/reverse reaction is endothermic/exothermic. If the forward reaction is endothermic, then increasing the temperature will shift the reaction in the forward direction, hence K will increase; if the forward reaction is exothermic, then K will decrease.
- Fri Jan 15, 2021 11:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure Rule
- Replies: 29
- Views: 1505
Re: Pressure Rule
We should only count the moles of gas molecules because they are the ones that will be affected by changes in pressure. By the way, solid and liquid play no role in the calculation of the equilibrium constant.
- Fri Jan 15, 2021 11:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Percent ionization
- Replies: 6
- Views: 227
Re: Percent ionization
Percent ionization is just the ratio of concentration of H+ ions ionized to the initial concentration of the acid.
Percent ionization=[H+]/[HA]*100%.
Percent ionization=[H+]/[HA]*100%.
- Fri Jan 15, 2021 11:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Molecular phases
- Replies: 6
- Views: 329
Re: Molecular phases
Aqueous(aq) is when you dissolve something into water to form a solution. For example, if you dissolve NaCl into water, you will get NaCl(aq). In contrast, liquid(l) simply means that a substance is in a purely liquid state. For example, H20(l) and C2H5OH(aq), which correspond to liquid water and li...
- Fri Jan 15, 2021 11:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling #3
- Replies: 5
- Views: 351
Re: Sapling #3
This is actually a very classical mistake when doing the ICE table! It should be (2x)^2, that is 4x^2, instead of 2x^2!
- Thu Jan 07, 2021 7:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs. Q
- Replies: 53
- Views: 2260
Re: K vs. Q
Q could be used at anytime of a reaction that is happening, it is just the ratio of products to reactants. However, K shall only be used when the reaction reaches equilibrium.
- Thu Jan 07, 2021 7:43 pm
- Forum: Ideal Gases
- Topic: Units of Temperature
- Replies: 82
- Views: 3998
Re: Units of Temperature
This one is actually important, we use Kelvin for the temperature in the Ideal Gas Equation.
- Thu Jan 07, 2021 7:39 pm
- Forum: Ideal Gases
- Topic: Temperature in Ideal Gas Law
- Replies: 14
- Views: 437
Re: Temperature in Ideal Gas Law
We actually use Kelvin for the temperature in Ideal Gas Equation.
- Thu Jan 07, 2021 7:35 pm
- Forum: Ideal Gases
- Topic: reversing reactions
- Replies: 83
- Views: 5588
Re: reversing reactions
For the reverse reaction, k of forward reaction will become 1/k in the reverse reaction.
- Thu Jan 07, 2021 7:33 pm
- Forum: Ideal Gases
- Topic: PV=nRT
- Replies: 74
- Views: 4842
Re: PV=nRT
P=pressure
V=volume
n=number of moles
R=constant(8.314J/(mol*K))
T=temperature
V=volume
n=number of moles
R=constant(8.314J/(mol*K))
T=temperature
- Sat Dec 12, 2020 4:17 am
- Forum: Lewis Acids & Bases
- Topic: Week 10 Sapling #6
- Replies: 7
- Views: 624
Re: Week 10 Sapling #6
NH3 is indeed a weak base as it could donate lone pair of electron. However, NaCl is a salt because when it dissolves in water, both ions will form ion-dipole attraction with water molecules. It is a mere physical process as nothing is really changed.
- Sat Dec 12, 2020 4:12 am
- Forum: Lewis Acids & Bases
- Topic: general conceptual question
- Replies: 9
- Views: 805
Re: general conceptual question
With regard to exam, you should really remember some of the most widely-known strong acid. These include: HClO4, HI, HBr, HCl, H2SO4, HNO3. To classify whether an acid is strong or weak, you should look at how easily the H atom would be lost. Weak A-H bond will result in a strong acid since the H at...
- Sat Dec 12, 2020 4:04 am
- Forum: Lewis Acids & Bases
- Topic: Textbook question
- Replies: 3
- Views: 230
Re: Textbook question
Like AlCl3, B in B(OH)3 also has empty orbital which means that it could accept electron pair from the OH- group, meaning it is a Lewis acid.
- Sat Dec 12, 2020 4:02 am
- Forum: Lewis Acids & Bases
- Topic: sapling #6
- Replies: 19
- Views: 970
Re: sapling #6
The carboxyl group could donate a H atom to form COO- and H+, so it is an acid, a weak acid though.
- Sat Dec 12, 2020 3:58 am
- Forum: Lewis Acids & Bases
- Topic: Water
- Replies: 63
- Views: 3028
Re: Water
Water can act as either an acid or a base, depending on the reactants.
- Fri Dec 04, 2020 10:40 pm
- Forum: Lewis Acids & Bases
- Topic: Classification of Lewis Acids and Bases
- Replies: 5
- Views: 292
Re: Classification of Lewis Acids and Bases
The main distinction would be that a Lewis acid has empty orbitals while a Lewis base has lone pairs of electrons. So that's why we say Lewis acid is electron acceptor while Lewis base is electron donor.
- Fri Dec 04, 2020 10:38 pm
- Forum: Lewis Acids & Bases
- Topic: Determining Lewis Acids and Bases
- Replies: 9
- Views: 609
Re: Determining Lewis Acids and Bases
If a species has an empty orbital, then it is a Lewis acid since it can accept lone pairs. If a species has lone pairs of electrons, then it is a Lewis base since it could donate electrons.
- Fri Dec 04, 2020 10:32 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Relative Acidity and stability
- Replies: 4
- Views: 305
Re: Relative Acidity and stability
Higher stability means a species is more easily to be formed. If the resulting anion isn't stable enough, then the dissociation won't be complete, which means that the acid is by definition, weak. The same logic applies to base as well.
- Fri Dec 04, 2020 10:27 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strength of Acids
- Replies: 8
- Views: 438
Re: Strength of Acids
HBr is the stronger acid because it has longer H-A bond, so weaker attraction between H and Br, thus proton is more easily dissociated.
- Fri Dec 04, 2020 10:25 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Acid strength and bond length
- Replies: 11
- Views: 2153
Re: Acid strength and bond length
For a structure of H-A, the longer the bond, the weaker the attraction, thus the stronger the acidity. In this case, HBr is more acidic than HCl.
- Sun Nov 29, 2020 8:38 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Polarity
- Replies: 16
- Views: 800
Re: Polarity
Not necessarily so, however, lone pair could indeed disrupt the symmetry of a molecule. For a molecule with lone pairs to be non-polar, the lone pairs must cancel out. For example, consider XeF4 where the lone pairs are opposite each other(one on top and one on bottom) and the covalent bonds form a ...
- Sun Nov 29, 2020 8:33 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: delocalized pi bond
- Replies: 8
- Views: 540
Re: delocalized pi bond
Delocalized pi bond is formed when 2 or more pi bonds are too close together so that they overlap. As a result, the electrons are free to move in the overlapped region. An example would be benzene where 3 pi bonds overlap, causing the formation of 6 delocalized electrons.
- Sat Nov 28, 2020 7:45 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Cisplatin Drug formation
- Replies: 3
- Views: 339
Re: Cisplatin Drug formation
My best guess is that cisplatin can bind to the N7 of the guanine molecule.
Check out the link below!
https://images.app.goo.gl/ZGgwc2LkrLssVM3j7
Check out the link below!
https://images.app.goo.gl/ZGgwc2LkrLssVM3j7
- Sat Nov 28, 2020 7:37 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Sigma and Pi Bonds
- Replies: 33
- Views: 1710
Re: Sigma and Pi Bonds
A triple bond contains 1 sigma bond and 2 pi bonds.
- Sat Nov 28, 2020 7:35 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: chelates
- Replies: 4
- Views: 388
Re: chelates
Typically, for a chelate, the ligand must not be a monodentate. This means the ligand must have multiple lone pairs so that it can form two or more coordinate bonds with the central atom.
- Sat Nov 21, 2020 4:03 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: determining molecular shape
- Replies: 10
- Views: 597
Re: determining molecular shape
Although CO bond is polar, CO2 molecule is linear in shape so that the dipoles cancel out. For SO2, it has two SO double bond and a lone pair on S atom. As a result, the shape is bent so that the dipoles can't cancel out.
- Sat Nov 21, 2020 3:46 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar Molecules and Dipole Moments
- Replies: 3
- Views: 258
Re: Polar Molecules and Dipole Moments
The double bond in this case matters because this way the C atoms can't rotate. Cis means that both of the chlorine atoms are on the same side of the compound. C-Cl bond is polar while C-H bond is non-polar, so the overall dipole moment will point to the chlorine side.
- Sat Nov 21, 2020 3:40 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook 2E #27c
- Replies: 6
- Views: 342
Re: Textbook 2E #27c
C-Cl bond is polar while C-H bond is non-polar. As a result, the dipoles can't cancel out.
- Sat Nov 21, 2020 3:39 am
- Forum: Hybridization
- Topic: Hybridization
- Replies: 7
- Views: 214
Re: Hybridization
Actually we combine 1 s orbital and 3 p orbitals to form 4 sp3 orbitals. Both of the electrons in the s orbitals are now in the sp3 orbitals.
- Sat Nov 14, 2020 2:01 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Force strength order
- Replies: 3
- Views: 327
Re: Force strength order
Order of intermolecular force from strongest to weakest: Hydrogen bond(strongest form of dipole-dipole interaction) dipole-dipole dipole-induced dipole interaction Van der Waal's forces Order of intramolecular forces: Ionic bond(assume solid ionic compound) covalent bond This answer is given by Khan...
- Sat Nov 14, 2020 1:51 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Identifying London Dispersion Forces
- Replies: 2
- Views: 217
Re: Identifying London Dispersion Forces
Molecules that are non-polar only experiences London Dispersion Forces. For example, hydrogen gas H2 and methane CH4.
- Fri Nov 13, 2020 11:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bent v. angular
- Replies: 27
- Views: 1531
Re: bent v. angular
From what I remembered from high school, bent and angular are the same thing where you have two bond pairs and two lone pairs on an atom.
- Fri Nov 13, 2020 11:06 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar or Nonpolar
- Replies: 13
- Views: 1084
Re: Polar or Nonpolar
Typically, symmetrical molecule is non-polar while asymmetrical molecule is polar because the dipoles can't cancel out.
- Fri Nov 13, 2020 11:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 11
- Views: 600
Re: VSEPR
Actually molecular shape is one very effective way to determine polarity. Typically, symmetrical molecule is non-polar while non-symmetrical molecule is polar because the dipoles can't cancel out.
- Sat Nov 07, 2020 1:30 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: 2D.9
- Replies: 6
- Views: 879
Re: 2D.9
Cation with greater charge density--greater charge/size ratio--has greater polarizing power. So polarizing power: Rb+<Sr2+<Be2+.
- Sat Nov 07, 2020 1:13 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing power of cations
- Replies: 2
- Views: 229
Re: Polarizing power of cations
I would say the charge density matters the most--that is the charge/size(surface area) ratio. Cation which has a high polarizing power will have a high charge density so that its charges are very concentrated. Typically, cation with a high charge and a small size has greater polarizing power.
- Sat Nov 07, 2020 1:04 am
- Forum: Coordinate Covalent Bonds
- Topic: Definition
- Replies: 17
- Views: 869
Re: Definition
A coordinate bond is formed when one atom donates a lone pair of electrons to an empty orbital of another atom. For example, an ammonia molecule has a lone pair of electron on the N atom, and a hydrogen ion has an empty orbital. As a result, the N atom donates the lone pair of electron to the hydrog...
- Sat Nov 07, 2020 1:02 am
- Forum: Coordinate Covalent Bonds
- Topic: Coordinate vs polar covalent
- Replies: 10
- Views: 2506
Re: Coordinate vs polar covalent
A coordinate bond is formed when one atom donates a lone pair of electrons to an empty orbital of another atom, so both electrons in the bond come from the same atom. However, a polar covalent bond just means that there is a big difference in electronegativity of the two atom, electrons are still sh...
- Sat Nov 07, 2020 12:55 am
- Forum: Coordinate Covalent Bonds
- Topic: Myoglobin
- Replies: 6
- Views: 1010
Re: Myoglobin
Myoglobins don't necessarily form hemoglobin, they are just structurally very similar to the sub-unit of hemoglobin. Also, each myoglobin can only bind to one oxygen molecule.
- Sat Oct 31, 2020 12:07 am
- Forum: Coordinate Covalent Bonds
- Topic: Coordinate covalent bonds
- Replies: 9
- Views: 806
Re: Coordinate covalent bonds
A regular covalent bond involves the sharing of electrons between 2 species. However, a coordinate bond is formed when one species donates a lone pair of electrons to an empty orbital of another species, which means that both electrons are supplied by the same species. We usually use an arrow to ind...
- Fri Oct 30, 2020 11:58 pm
- Forum: Ionic & Covalent Bonds
- Topic: ligands
- Replies: 7
- Views: 1688
Re: ligands
Ligand is a species that have one or more lone pairs of electrons available to donate to a central metal ion, forming coordinate bonds.
- Fri Oct 30, 2020 8:38 am
- Forum: Ionic & Covalent Bonds
- Topic: Nonpolar and polar
- Replies: 16
- Views: 1131
Re: Nonpolar and polar
Firstly, you need to check if some of the atoms are more electronegative than others. Secondly, you need to see if the dipole cancels out. For example, CO2 has polar bonds, but the molecular is non-polar.
- Fri Oct 30, 2020 8:36 am
- Forum: Ionic & Covalent Bonds
- Topic: intermolecular vs intramolecular
- Replies: 17
- Views: 1972
Re: intermolecular vs intramolecular
Intermolecular bonding is the bond formed between molecules, such as hydrogen bond and Van der Waal's forces. Intramolecular bonding refers to the bond formed within a molecule, such as covalent bond.
- Fri Oct 30, 2020 8:34 am
- Forum: Ionic & Covalent Bonds
- Topic: hydrogen
- Replies: 19
- Views: 989
Re: hydrogen
For hydrogen bond, H atom must be bonded to a very electronegative atom such as N, O, or F so that the only electron of hydrogen atom is nearly completely pulled over to the lone pair electronegative atom.
- Sun Oct 25, 2020 6:11 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Multi-Electron Atoms
- Replies: 3
- Views: 178
Re: Multi-Electron Atoms
Firstly, there is attraction between the positively charged nucleus and the electrons. Secondly, there is repulsion forces between electrons in the same orbital because same charge repels each other. Thirdly, there is the shielding effect caused by the repulsion from the inner electron shells. Elect...
- Fri Oct 23, 2020 7:22 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: delta V
- Replies: 4
- Views: 228
Re: delta V
For example, if speed is 10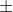 3m/s, you could either multiply 3 by 2 or use maximum value to minus the minimum value, that is 13-7=6m/s. Both way is correct.
3m/s, you could either multiply 3 by 2 or use maximum value to minus the minimum value, that is 13-7=6m/s. Both way is correct.
- Fri Oct 23, 2020 7:15 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Problem 1 B.27
- Replies: 2
- Views: 91
Re: Problem 1 B.27
To calculate the uncertainty in position, you firstly have to determine the uncertainty in momentum. The speed is 5 \pm 5m/s, so the uncertainty in speed is 10-0=10m/s. We then calculate the uncertainty in momentum using p=mv. Finally, we plug the value of momentum into Heisenberg's equation, and th...
- Fri Oct 23, 2020 8:21 am
- Forum: DeBroglie Equation
- Topic: Sapling Question #10
- Replies: 8
- Views: 616
Re: Sapling Question #10
Firstly, you need to calculate the mass of a single fluorine molecule. To do that, you could divide the molecular mass(this is in g) of fluorine molecule by the Avogadro number. Please note that you need to convert g to kg! Once you have the mass of a single fluorine molecule, you could use the form...
- Fri Oct 23, 2020 8:11 am
- Forum: DeBroglie Equation
- Topic: Sapling Homework - electron affinity
- Replies: 9
- Views: 470
Re: Sapling Homework - electron affinity
So for calculating energy of photons, you could use the equation E=h*f where frequency of light could be determined via c=f*wavelenth.
For calculating energy of electrons, you could firstly calculate the momentum of electron via p=h/wavelength, and then apply the value of p to the equation p^2/2m=Ek.
For calculating energy of electrons, you could firstly calculate the momentum of electron via p=h/wavelength, and then apply the value of p to the equation p^2/2m=Ek.
- Fri Oct 23, 2020 8:06 am
- Forum: DeBroglie Equation
- Topic: Rearranging De Broglie Equation
- Replies: 17
- Views: 2305
Re: Rearranging De Broglie Equation
According to De Broglie's equation: wavelength=planck constant/momentum.
Momentum=mass*velocity
As a result, wavelength=planck constant/(mass*velocity), so velocity=planck constant/(mass*wavelength).
Momentum=mass*velocity
As a result, wavelength=planck constant/(mass*velocity), so velocity=planck constant/(mass*wavelength).
- Thu Oct 15, 2020 9:24 pm
- Forum: DeBroglie Equation
- Topic: Wavelengths and DeBroglie
- Replies: 7
- Views: 202
Re: Wavelengths and DeBroglie
According to De Broglie equation,any object with a mass and a velocity(that is to say, any object with momentum)can be considered as a wave. However, since baseball has a relatively large mass, the wavelength will be too small to be detectable. Let's say the baseball weighs 145g and moves at 40m/s. ...
- Thu Oct 15, 2020 9:12 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect
- Replies: 3
- Views: 249
Re: Photoelectric Effect
For part A: kinetic energy is just Ek=1/2 m v^2
For part B: note the unit here--Kj/mol, so you need to divide the work function by the Avogadro's constant, and the unit should be Jk
For part C: firstly you add work function and kinetic energy together. Then you divide the sum by the Planck constant.
For part B: note the unit here--Kj/mol, so you need to divide the work function by the Avogadro's constant, and the unit should be Jk
For part C: firstly you add work function and kinetic energy together. Then you divide the sum by the Planck constant.
- Wed Oct 14, 2020 8:32 am
- Forum: Properties of Light
- Topic: High Frequency = Energy?
- Replies: 4
- Views: 296
Re: High Frequency = Energy?
According to the equation E=h*f, higher frequency means each photon has higher energy. Intensity is essentially number of photons, so if you increase the intensity of the light, the light beam will get brighter. To increase the amplitude, or intensity of a light wave, in reality you just need to inc...
- Wed Oct 14, 2020 8:27 am
- Forum: Properties of Light
- Topic: Electric Field Wave vs Magnetic Field Wave
- Replies: 3
- Views: 173
Re: Electric Field Wave vs Magnetic Field Wave
Fields are essentially vectors which have directions. Direction of electric field and direction of magnetic field are perpendicular to each other--they are oscillating at 90 degrees to each other.
- Wed Oct 14, 2020 8:24 am
- Forum: Properties of Light
- Topic: What exactly is Φ?
- Replies: 16
- Views: 1093
Re: What exactly is Φ?
Work function is the threshold energy--the minimum amount of energy needed to release electrons from a metal surface. Too calculate it, you need to know exactly at which frequency of light does the electrons get released. Then simply use the equation: E=h*f. If the energy of light is big enough to r...
- Wed Oct 07, 2020 9:48 pm
- Forum: Limiting Reactant Calculations
- Topic: Question about Molar Ratios with Limiting Reactants
- Replies: 8
- Views: 314
Re: Question about Molar Ratios with Limiting Reactants
You must have 2 units of B and 1 unit of A in order for the reaction to proceed. So if you have fewer moles of B than A, A is then clearly in excess. What you really need to do is doubling the mole of A to see if it equals number of moles of B. If it is more than moles of B, than B is the limiting r...
- Wed Oct 07, 2020 9:42 pm
- Forum: Balancing Chemical Reactions
- Topic: Combustion
- Replies: 14
- Views: 468
Re: Combustion
Combustion is essentially a oxidation reaction because you use "oxygen" to burn something--to oxidize something. However, with regard to burning, you don't necessarily have to use oxygen. You just need to make something burn--a lot of chemicals can burn things.
- Tue Oct 06, 2020 6:29 am
- Forum: Balancing Chemical Reactions
- Topic: Conservation of electrons/protons
- Replies: 7
- Views: 736
Re: Conservation of electrons/protons
Electrons and protons are charged so if they are not conserved, the number of charges of a reaction would change which clearly violates the "conservation of charge" law.
- Tue Oct 06, 2020 6:25 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Accuracy vs Precision
- Replies: 20
- Views: 689
Re: Accuracy vs Precision
Accuracy is how close you are to the real/true value. Precision is how close your experimental data is to each other. For example, let's say you want to measure the length of your pencil which is 15cm long and you measure 3 times. If you get 15.1cm, 15.0cm, 14.9cm, then you are both accurate and pre...

