Search found 108 matches
- Mon Mar 15, 2021 9:56 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Calorimeter
- Replies: 6
- Views: 611
Re: Calorimeter
I don’t know if this is correct, but I just use q = C(delta)T.
- Mon Mar 15, 2021 9:54 am
- Forum: First Order Reactions
- Topic: Half Life Unit
- Replies: 38
- Views: 1649
Re: Half Life Unit
If you’re solving for half life, then the units really depend on the units of k. Just remember to write out your units when you do the math.
It can be in seconds, minutes, hours, whatever the problem (or answer choices) say it is.
It can be in seconds, minutes, hours, whatever the problem (or answer choices) say it is.
- Mon Mar 15, 2021 9:52 am
- Forum: Zero Order Reactions
- Topic: Overall order of the reaction
- Replies: 45
- Views: 2084
Re: Overall order of the reaction
The overall order of a reaction is the sum of the orders of the reaction’s elementary steps.
- Mon Mar 15, 2021 9:49 am
- Forum: General Science Questions
- Topic: Final thoughts
- Replies: 28
- Views: 4924
Re: Final thoughts
I thought the final was a lot easier than the one from 14A. I also had an easier time with the final compared to midterm 2, which I thought was the hardest exam this quarter. Maybe it’s because I’m just not as strong in thermo relative to equilibrium, redox, & kinetics.
- Mon Mar 15, 2021 9:47 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Cell Diagrams
- Replies: 2
- Views: 306
Re: Cell Diagrams
You include Pt when there isn’t a solid electrode in the cell where a reduction or oxidation reaction is occurring.
Pt is an inert electrode and won’t be involved in the redox reaction.
Pt is an inert electrode and won’t be involved in the redox reaction.
- Sat Mar 13, 2021 3:11 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts and intermediates
- Replies: 10
- Views: 639
Re: Catalysts and intermediates
This is how I reason through it: Usually, an intermediate is produced by one of the elementary steps and is consumed as a reactant of another elementary step (appears in the product side of one step, appears in reactant side of following step). This intermediate was consumed. A catalyst first comes ...
- Wed Mar 10, 2021 7:20 am
- Forum: Student Social/Study Group
- Topic: Calculators
- Replies: 52
- Views: 3539
Re: Calculators
I think the scientific calculators are preferred, but as long as you aren't using the graphing calculator-exclusive functions (like storing equations, etc.), then it's fine. During exams people show their graphing calculators when showing their other papers and the TAs seem fine with it.
- Wed Mar 10, 2021 7:19 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Initial Rates = Maximum rates [ENDORSED]
- Replies: 9
- Views: 867
Re: Initial Rates = Maximum rates [ENDORSED]
I think the initial rate is considered the maximum rate because there is the most reactant available. Once the reaction starts occurring, the amount of reactant decreases. During the initial rate, there is very little product formed, so the reverse reaction is considered to not be taking place (of c...
- Wed Mar 10, 2021 7:12 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Exergonic vs Exothermic [ENDORSED]
- Replies: 4
- Views: 411
Re: Exergonic vs Exothermic [ENDORSED]
Exergonic and endergonic usually refer to Gibbs free energy.
Endothermic & exothermic refer to enthalpy.
I remember it because exergonic & endergonic have "g" like Gibbs while endothermic & exothermic have "th" like enthalpy.
Endothermic & exothermic refer to enthalpy.
I remember it because exergonic & endergonic have "g" like Gibbs while endothermic & exothermic have "th" like enthalpy.
- Wed Mar 10, 2021 7:10 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 11702
Re: What was your favorite chem topic?
I think I enjoyed chemical equilibrium at the beginning of 14B the most. Second is probably Lewis Structures and things related to that.
- Sun Mar 07, 2021 10:14 pm
- Forum: Administrative Questions and Class Announcements
- Topic: FINAL EXAM QUESTIONS
- Replies: 40
- Views: 2799
Re: FINAL EXAM QUESTIONS
Based on past experience with Chem 14A, there is probably going to be some problems from the textbook, but the majority will not be directly from the textbook. That being said, doing textbook problems are still a really good way to study for the final.
- Sun Mar 07, 2021 9:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalysts
- Replies: 4
- Views: 307
Re: Catalysts
I think Ian Wheeler is correct about poisoning. From my understanding, enzymes can be inhibited by poisons when those poisons act as competitive or noncompetitive inhibitors. Competitive inhibitors bind to the active site of enzymes (site on enzyme where they catalyze a reaction, thus blocking subst...
- Sun Mar 07, 2021 8:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Notation
- Replies: 5
- Views: 433
Re: Cell Notation
I think the solid compound is the electrode. Example: Cu(s) | Cu2+(aq) || Fe3+(aq), Fe2+(aq) | Pt(s) This represents the redox reaction Fe3+(aq) + Cu(s) → Cu2+(aq) + Fe2+(aq). On the left side, Cu(s) is being oxidized to Cu2+(aq). Cu(s) on the left and Cu2+(aq) on the right represents the direction ...
- Sun Mar 07, 2021 8:48 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E° vs. E and G° vs. G
- Replies: 25
- Views: 1083
Re: E° vs. E and G° vs. G
Yes, you are correct. Standard conditions are when something occurs at 1 atm or 1 M and at 298 K or 25°C. The E° and G° are used as standards because these values don't actually change. They serve as a sort of "base" or comparison to measure E & G values when certain factors are change...
- Sun Mar 07, 2021 8:41 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 17163
Re: How do you deal with burnout?
I don't know if this works for everyone, but I usually feel burnt out if I do a lot of work in one sitting without break (sometimes I get a burst of motivation and ride that wave until I can't work any longer). Afterwards I am unmotivated to work for a very long time. Especially because everything's...
- Sun Feb 28, 2021 11:37 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: K meaning
- Replies: 29
- Views: 2290
Re: K meaning
It is located on the very top right on the Constants and Formulas Sheet.
- Sun Feb 28, 2021 9:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridges
- Replies: 41
- Views: 3220
Re: Salt Bridges
Salt bridges help keep electrons moving from the anode to the cathode. Spectator ions move from one solution to another via the salt bridge. This prevents the charge from evening out across both sides of the cell, which would prevent electrons from continuously flowing in one direction.
- Sun Feb 28, 2021 9:48 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #18 Clarification
- Replies: 2
- Views: 190
Sapling Week 7/8 #18 Clarification
Hello.
The problem involves iron(III) oxide trihydrate. What is a hydrate and do we need to determine what hydrates' chemical formulas are for the test?
The problem involves iron(III) oxide trihydrate. What is a hydrate and do we need to determine what hydrates' chemical formulas are for the test?
- Sun Feb 28, 2021 9:44 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs E naught
- Replies: 36
- Views: 1734
Re: E vs E naught
E° is just E under standard conditions, which are 1 M, 1 atm, & at 25°C (or 298 K).
- Thu Feb 25, 2021 12:16 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing vs Reducing
- Replies: 55
- Views: 2672
Re: Oxidizing vs Reducing
I just remember that in a redox reaction, if one species is being reduced, another is being oxidized. OILRIG (oxidation is loss, reduction is gain) If Species X is being reduced, it is causing Species Y to be oxidized because X gains the electrons that Y lost. What is being reduced is the oxidizing ...
- Sun Feb 21, 2021 11:13 pm
- Forum: Ideal Gases
- Topic: Temperature
- Replies: 99
- Views: 7031
Re: Temperature
If it isn't given in a problem, you usually assume it's 298 K (or 25°C). I used to wonder this too but that is how it is in a lot of the Sapling textbook questions. If there is any ambiguity during an exam and you just want to be safe, I'm sure your TA will clarify it anyway.
- Fri Feb 19, 2021 1:18 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Textbook 4.37
- Replies: 1
- Views: 182
Textbook 4.37
The question is: Under what conditions, if any, does the sign of each of the following quantities provide a criterion for assessing the spontaneity of a reaction? (a) ΔG°; (b) ΔH°; (c) ΔS°; (d) ΔS(total). Answers: ∆G° < 0 ∆H°: unable to tell ∆S°: unable to tell ∆S(total) > 0 What is the difference b...
- Thu Feb 18, 2021 7:15 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Entropies of Solid Products vs. Solid Reactants?
- Replies: 2
- Views: 286
Entropies of Solid Products vs. Solid Reactants?
The textbook problem 4H.11 asks you to find ∆S for reactions using the reactants' & products' standard molar entropies. For the reaction: 4KClO_{3(s)} \rightarrow 3KClO_{4(s)} + KCl_{(s)} ∆S° for the reaction = -36.81 J/(K•mol) We have to explain why the entropy is decrea...
- Thu Feb 18, 2021 1:21 am
- Forum: Calculating Work of Expansion
- Topic: Textbook Problem 4A.3
- Replies: 3
- Views: 260
Textbook Problem 4A.3
For part a, you find work by multiplying pressure, volume, and another value.
w = -(2.00 atm)(-0.14 L)(101.325 J/(L•atm)) = 28 J
What is the 101.325 J/(L•atm) value? I can't seem to find it in my notes. And why do we multiply it with the pressure & volume?
w = -(2.00 atm)(-0.14 L)(101.325 J/(L•atm)) = 28 J
What is the 101.325 J/(L•atm) value? I can't seem to find it in my notes. And why do we multiply it with the pressure & volume?
- Wed Feb 17, 2021 8:45 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: R gas constant and kPa or Pa
- Replies: 9
- Views: 3282
R gas constant and kPa or Pa
I was going over the Sapling homework and problem #5 gives pressure in kPa.
What gas constant would we use when given that unit of pressure? Do we use 8.314 J/(K•mol)?
Also, why is the 8.314 J/(K•mol) the only value of R that doesn't have a unit of pressure in the units?
What gas constant would we use when given that unit of pressure? Do we use 8.314 J/(K•mol)?
Also, why is the 8.314 J/(K•mol) the only value of R that doesn't have a unit of pressure in the units?
- Wed Feb 17, 2021 8:02 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Sapling Week 5/6 #7 Feedback Question
- Replies: 1
- Views: 128
Sapling Week 5/6 #7 Feedback Question
So I was completing a Sapling homework problem where we had to list compounds from most to least ordered according to ∆S(vap). I got it wrong the first time (got it right the 2nd time), but I was confused about this text from the feedback: "A larger molar entropy of vaporization, which correlat...
- Sun Feb 14, 2021 7:39 pm
- Forum: Calculating Work of Expansion
- Topic: irreversible vs reversible
- Replies: 14
- Views: 735
Re: irreversible vs reversible
Reversible expansions occur slower than irreversible expansions. If an expansion is "quick" or "sudden", then it's most likely irreversible. If the expansion is "slow" or "gradual", then it is most likely reversible.
- Sun Feb 14, 2021 7:36 pm
- Forum: Van't Hoff Equation
- Topic: Celcius vs Kelvin for T1 and T2
- Replies: 84
- Views: 7118
Re: Celcius vs Kelvin for T1 and T2
I usually always convert to Kelvin because most of our constants use Kelvin. Unless a constant given specifically uses Celsius or the question asks you to use/give your answer in Celsius, just use Kelvin.
- Sun Feb 14, 2021 7:31 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Sapling 3
- Replies: 13
- Views: 606
Re: Sapling 3
This is how I worked it out (I'll use water as a place holder): Solid to Liquid: +∆H & +∆S energy + H2O(s) → H2O(l) Ice becomes liquid when energy (heat) is added to it. This would mean the reaction is endothermic (+∆H). When a solid becomes a liquid, entropy increases because the # of possible ...
- Sun Feb 14, 2021 7:24 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Likeliness to form products/reactants
- Replies: 27
- Views: 988
Re: Likeliness to form products/reactants
For A + B → C:
If ∆G < 0 (is negative), then the reaction is spontaneous. This means the forward reaction is favored (producing C).
If ∆G > 0 (is positive), then the reaction isn't spontaneous (in the forward direction). This means the reverse reaction is favored (producing A & B).
If ∆G < 0 (is negative), then the reaction is spontaneous. This means the forward reaction is favored (producing C).
If ∆G > 0 (is positive), then the reaction isn't spontaneous (in the forward direction). This means the reverse reaction is favored (producing A & B).
- Sun Feb 14, 2021 7:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: struggling
- Replies: 73
- Views: 4109
Re: struggling
Hello. The UA sessions are pretty helpful. Even if you can't catch one of their sessions, it's helpful to do the problems they go over. I think usually someone uploads the worksheets after. Make sure not to overwork yourself before the exam. I've done that in the past trying to get lots of studying ...
- Sat Feb 06, 2021 10:39 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Key points of First Law
- Replies: 8
- Views: 248
Re: Key points of First Law
I think that Conservation of Energy is just remembering that energy is moving between system & surrounding. Energy doesn't just "disappear" (it can escape into surroundings).
- Sat Feb 06, 2021 10:32 pm
- Forum: Student Social/Study Group
- Topic: Study tips for thermo
- Replies: 14
- Views: 883
Re: Study tips for thermo
I don't know how much it will help you, but here are some things that I do: Usually looking at my notes for lectures is overwhelming since it's multiple pages. I make a 1-page sum-up of what we learned per week or per outline. If I don't get ideas from the lecture or from the textbook, I ask Chemist...
- Sat Feb 06, 2021 10:29 pm
- Forum: General Science Questions
- Topic: Careless Mistakes
- Replies: 54
- Views: 3892
Re: Careless Mistakes
Hello. I don't know if this helps, but after doing lots of practice problems, I have a good idea of what a "reasonable" number would be for my calculation. Like if I was converting grams to moles, usually I wouldn't get a number like 5,000 moles. When I look at intermediate values while ca...
- Sat Feb 06, 2021 10:20 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: R Constant
- Replies: 91
- Views: 6811
Re: R Constant
Whichever R value you use depends on the units of the other values you are given in a problem.
- Sat Feb 06, 2021 10:18 pm
- Forum: Student Social/Study Group
- Topic: Study routine.
- Replies: 51
- Views: 2747
Re: Study routine.
I would try to do lots of practice problems throughout the weeks before the midterm (not just doing them all right before the midterm; I've done it and for me at least it is not as effective). I think that if you are not doing well when reading the textbook, you should try to listen to videos (Khan ...
- Sat Feb 06, 2021 10:11 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Sapling Week 3/4 #19
- Replies: 6
- Views: 221
Sapling Week 3/4 #19
Hello. Here is the problem: A constant‑volume calorimeter was calibrated by carrying out a reaction known to release 1.11 kJ of heat in 0.600 L of solution in the calorimeter (q=−1.11 kJ) , resulting in a temperature rise of 2.10 ∘C . In a subsequent experiment, 300.0 mL of 0.10 M HClO2(aq) and 300....
- Sat Feb 06, 2021 10:04 pm
- Forum: Student Social/Study Group
- Topic: Silly Mistakes?
- Replies: 72
- Views: 6502
Re: Silly Mistakes?
I never really thought about "silly mistakes" in this way before. Thank you for sharing this. I think a lot of people (including myself) are too hard on ourselves for those types of mistakes.
- Wed Feb 03, 2021 7:51 pm
- Forum: Ideal Gases
- Topic: Units for K
- Replies: 29
- Views: 1300
Re: Units for K
I think because it's a ratio, it has no units. Not 100% sure if that's the correct explanation but that's what a teacher told me in high school.
- Sun Jan 31, 2021 11:15 pm
- Forum: General Science Questions
- Topic: Midterm 1 Reactions
- Replies: 70
- Views: 5123
Re: Midterm 1 Reactions
I thought it was similar to the midterms from Lavelle's Chem 14A, so I wasn't too surprised. Of course, still anxious to see my score. I hope I didn't make a dumb mistake that I wouldn't have made if I was going slower, but that's just how tests go.
- Thu Jan 28, 2021 1:32 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acid & Base Dissociation Values
- Replies: 1
- Views: 82
Acid & Base Dissociation Values
When asked to compare the strengths of 2 acids or bases, are we going to be given pKa/pKb or Ka/Kb values? For example, here is the problem that made me think of this question: Textbook Question 6C.17 Which is the stronger base, the hypobromite ion, BrO−, or morphine, C17H19O3N? Justify your answer....
- Thu Jan 28, 2021 1:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 6C.15 [ENDORSED]
- Replies: 2
- Views: 302
Textbook 6C.15 [ENDORSED]
The question is: The pKa of HIO(aq), hypoiodous acid, is 10.64 and that of HIO3(aq), iodic acid, is 0.77. Account for the difference in strength. The answer talks about how there are more electronegative O atoms around the central atom in HIO3, which makes the central atom's oxidation number greater...
- Mon Jan 25, 2021 3:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5.35
- Replies: 1
- Views: 70
Textbook Problem 5.35
Hello.
Part B of this problem asks you to calculate K for the reaction.
In the solution to the problem, when finding K you take the pressures in the graph and divide by 100. Why do you do that?
Part B of this problem asks you to calculate K for the reaction.
In the solution to the problem, when finding K you take the pressures in the graph and divide by 100. Why do you do that?
- Mon Jan 25, 2021 3:12 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Textbook Question 5J.5, element "D"?
- Replies: 2
- Views: 150
Textbook Question 5J.5, element "D"?
Hello.
Part d of 5J.5 asks about the reaction:
2HD(g) ⇌ H2(g) + D2(g)
What element is "D" in this problem? Maybe I'm just missing it but I can't find it on the periodic table?
Part d of 5J.5 asks about the reaction:
2HD(g) ⇌ H2(g) + D2(g)
What element is "D" in this problem? Maybe I'm just missing it but I can't find it on the periodic table?
- Sat Jan 23, 2021 10:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids in Ice Tables
- Replies: 11
- Views: 1044
Re: Solids in Ice Tables
From what I remember, you can disregard the concentrations of solids & liquids in ICE tables.
- Sat Jan 23, 2021 10:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How to fill in 2nd row of ICE table?
- Replies: 25
- Views: 899
Re: How to fill in 2nd row of ICE table?
I think you need to determine what direction the reaction is going. In many cases, if you only start with reactants, then the reaction would create products. If you are given a K value and have concentrations for all reactant & product species, you can calculate Q and compare the two. If Q > K, ...
- Sat Jan 23, 2021 9:56 pm
- Forum: Student Social/Study Group
- Topic: Midterm Study Tips
- Replies: 41
- Views: 1869
Re: Midterm Study Tips
When doing UA, textbook, or online Sapling problems, I would do the hard problems and progressing to the easier ones. If you can't do the hardest problem, go to the second hardest, and onward. In my opinion, you waste less time on simple problems that you've already gotten the hang of.
- Sat Jan 23, 2021 9:54 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: amphoteric vs amphiprotic
- Replies: 10
- Views: 343
Re: amphoteric vs amphiprotic
Amphoteric means that a species can react as a base or acid.
Amphiprotic means that a species can donate or accept a proton (an H+ ion).
From my understanding, all amphiprotic species are technically amphoteric, but not all amphoteric species count as amphiprotic.
Amphiprotic means that a species can donate or accept a proton (an H+ ion).
From my understanding, all amphiprotic species are technically amphoteric, but not all amphoteric species count as amphiprotic.
- Sun Jan 17, 2021 1:59 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Amphiprotic
- Replies: 8
- Views: 375
Re: Amphiprotic
Hello.
Amphiprotic species can act as a Brønsted-Lowry acid or base, meaning it can act as a proton (H+) donor & acceptor. One example is water, as it can donate a proton (OH-) or accept a proton (H3O+).
Hope that helps.
Amphiprotic species can act as a Brønsted-Lowry acid or base, meaning it can act as a proton (H+) donor & acceptor. One example is water, as it can donate a proton (OH-) or accept a proton (H3O+).
Hope that helps.
- Sun Jan 17, 2021 1:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Chart with Gas Pressures
- Replies: 8
- Views: 329
ICE Chart with Gas Pressures
Hello.
So when we are given Kp and pressures of compounds in bar, do we just use those pressures in the ICE chart instead of molarity amounts? Adding onto that, do we need to convert to atm?
So when we are given Kp and pressures of compounds in bar, do we just use those pressures in the ICE chart instead of molarity amounts? Adding onto that, do we need to convert to atm?
- Sun Jan 17, 2021 1:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5I.25
- Replies: 1
- Views: 69
Re: Textbook Problem 5I.25
Hello. I think your K equation: (0.02 + x)(0.03 + x) / (0.02 - x)(0.04 - x) = 85.0 is correct. Your quadratic formula 54x^2 - 5.6x + 0.06794 = 0 differs from the correct one: 84x^2 - 5.15x + 0.0674 = 0 There must have been some algebra mistake between those steps which got you to the wrong answer, b...
- Sun Jan 10, 2021 10:49 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q>K
- Replies: 10
- Views: 373
Re: Q>K
I think one example of when Q > K is when the reaction is not quite at equilibrium yet so there are still products that are forming reactants. Because K & Q = [Products]/[Reactants], if Q > K, then there are more products that are still forming those reactants. This can also occur if more produc...
- Sun Jan 10, 2021 10:46 am
- Forum: Administrative Questions and Class Announcements
- Topic: 14B Midterm Content
- Replies: 4
- Views: 348
14B Midterm Content
Hi. For our final & midterms, are we only being tested on content that we learned in 14B or also 14A? I know that some foundation from 14A will be utilized when understanding certain concepts from 14B, but for example, will topics related to quantum mechanics be on those exams? I don't remember ...
- Sun Jan 10, 2021 10:41 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium constant between 10^-3 and 10^3
- Replies: 6
- Views: 701
Re: Equilibrium constant between 10^-3 and 10^3
I think Lavelle said that if 10^-3 < K < 10^3 (intermediate values of K), then neither reactants nor products are strongly favored in that reaction (from the lecture on 1/6).
- Sun Jan 10, 2021 10:39 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Q
- Replies: 7
- Views: 258
Re: K vs Q
Hello. I'm pretty sure both K & Q are found by dividing product concentrations (to the power of their coefficients) by the reactant concentrations (to the power of their coefficients). The difference is that K uses equilibrium concentrations. When you are given concentrations of reactants & ...
- Mon Jan 04, 2021 4:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 1 Problem #1
- Replies: 5
- Views: 354
Sapling Week 1 Problem #1
The question is: "Select all of the true statements regarding chemical equilibrium:" Two of the options are: 1. "The concentrations of the reactants and products are equal" 2. "The concentrations of the reactants and products remain constant" #2 is true and #1 is false....
- Wed Dec 16, 2020 6:41 pm
- Forum: General Science Questions
- Topic: final timing
- Replies: 25
- Views: 1575
Re: final timing
I usually finish exams (especially multiple choice ones) with a moderate amount of time to spare, but this exam went by pretty fast. But I kind of expected it because it was a final and braced myself for the possibility that I may not finish. Personally, the questions with finding empirical formula ...
- Wed Dec 16, 2020 6:34 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why isn't HF a strong acid?
- Replies: 23
- Views: 7482
Re: Why isn't HF a strong acid?
F is very electronegative, so it does not want to dissociate with the H atom. This is why it is not a strong acid.
Among HCl, HBr, and HI, HI is the strongest acid because it gives up protons the easiest. The bond for HI is the longest, so it is the weakest.
Among HCl, HBr, and HI, HI is the strongest acid because it gives up protons the easiest. The bond for HI is the longest, so it is the weakest.
- Wed Dec 09, 2020 7:16 pm
- Forum: Hybridization
- Topic: What is s-character?
- Replies: 1
- Views: 243
What is s-character?
Textbook problem 2F.15 asks "Noting that the bond angle of an sp3 hybridized atom is 109.58 and that of an sp2 hybridized atom is 1208, do you expect the bond angle between two hybrid orbitals to increase or decrease as the s-character of the hybrids is increased?" The answer is "As t...
- Tue Dec 08, 2020 4:21 pm
- Forum: Industrial Examples
- Topic: What are these?
- Replies: 2
- Views: 682
Re: What are these?
I don't remember many examples, but Lavelle did mention 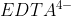 , which removes metals from solutions (from lecture on 11/25).
, which removes metals from solutions (from lecture on 11/25).
- Tue Dec 08, 2020 3:58 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Sapling #7
- Replies: 3
- Views: 242
Re: Sapling #7
Memorizing if salts are acidic/basic/neutral seems pretty difficult considering there could be other salts on the exam that you didn't prepare for. From my understanding, you need to determine what acid & base reacted to produce that salt. Looking at the strengths of both reactants will determin...
- Tue Dec 08, 2020 3:52 pm
- Forum: Significant Figures
- Topic: Things to remember for final?
- Replies: 20
- Views: 1344
Re: Things to remember for final?
You only really need to know which acids/bases are strong and if you know the general rules for whether something is an acid or base, you can rule out if it's strong. There's too many weak acids/bases to memorize, but you can remember the strong ones easily.
- Tue Dec 08, 2020 3:49 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: -OH vs OH-
- Replies: 36
- Views: 1436
Re: -OH vs OH-
It's the same thing. In general, 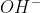 is how you'd normally write it when writing a chemical equation.
is how you'd normally write it when writing a chemical equation. 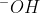 is used more when writing diagrams or mechanisms to emphasize that the negative charge is from the oxygen, not the hydrogen.
is used more when writing diagrams or mechanisms to emphasize that the negative charge is from the oxygen, not the hydrogen.
- Wed Dec 02, 2020 9:55 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis Acids & Bases
- Replies: 12
- Views: 1177
Re: Lewis Acids & Bases
Lewis Acids are electron acceptors (I remember that because acids and acceptor both start with 'a'). These acids are electron deficient. I usually remember that if it has a positive charge, it can accept an electron (cations). Lewis Bases are electron donors. These bases may have negative charges (a...
- Wed Dec 02, 2020 9:42 pm
- Forum: Lewis Acids & Bases
- Topic: Ligands
- Replies: 6
- Views: 498
Re: Ligands
Ligands are ions/molecules that form coordinate covalent bonds by donating both electrons involved in the covalent bond. Because it donates electron pair(s), if you draw out the Lewis structure, it should have lone electron pairs. It is also generally a nonmetal in a coordination compound.
- Wed Dec 02, 2020 9:38 pm
- Forum: Student Social/Study Group
- Topic: Final Exam Study Tips
- Replies: 48
- Views: 2614
Re: Final Exam Study Tips
My plan is to do the textbook problems listed in the outline. Since this final is cumulative, you also have to find time to review material from the previous 2 midterms. I suggest doing some of the harder textbook problems to review past content because I think it was mentioned some difficult questi...
- Wed Dec 02, 2020 9:33 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sapling Week 9 HW Question 2
- Replies: 11
- Views: 631
Re: Sapling Week 9 HW Question 2
Hello. When determining coordination number, the coordination sphere consists of the elements in brackets. At first I thought the coordination number was 2, but that's because I was looking at Ba, which was outside the brackets. Fe is the central atom and has 4 Br atoms bound to it, which means the ...
- Sun Nov 29, 2020 5:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook Problem 2E5
- Replies: 3
- Views: 197
Re: Textbook Problem 2E5
a) The shape of the 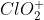 ion is bent/angular.
ion is bent/angular.
b) The bond angle between the two O atoms are slightly less than 120 degrees due to the presence of a lone electron pair.
b) The bond angle between the two O atoms are slightly less than 120 degrees due to the presence of a lone electron pair.
- Sat Nov 28, 2020 7:57 pm
- Forum: Hybridization
- Topic: Multiple Bonds with Hybridization
- Replies: 6
- Views: 350
Re: Multiple Bonds with Hybridization
I'm not sure if this is the right way to explain this, but when determining hybridization of an atom you look at the number of atoms it's attached to and the number of lone pairs that it has. The number of bonds between the atoms does not affect it. I am assuming Molecule #1 has a central atom with ...
- Sat Nov 28, 2020 7:11 pm
- Forum: Octet Exceptions
- Topic: Textbook Question 2.61
- Replies: 3
- Views: 347
Re: Textbook Question 2.61
I am not sure if this is correct, but I tried looking at alternate structures where the lone electron is not on the C. The structure where the lone electron is on C is the favored structure because that structure has all atoms with a formal charge of 0. If the lone electron is on an oxygen, the form...
- Sat Nov 28, 2020 7:02 pm
- Forum: Student Social/Study Group
- Topic: Midterm/Final Success?
- Replies: 17
- Views: 838
Re: Midterm/Final Success?
In my experience, I do all of the textbook problems in the outline and the UA problems on my own. When I have questions about certain topics, I utilize both this forum and chemistry videos on YouTube (I used these in high school and they really helped me). It's helpful to compile a list of condensed...
- Tue Nov 17, 2020 4:03 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: d vs s orbitals
- Replies: 7
- Views: 1054
Re: d vs s orbitals
Hello. From how I understand it: 1. Electrons are added to the 4s orbitals before the 3d orbitals because the 4s orbitals are lower in energy when both are unoccupied. The 4s and 3d orbitals are very close in energy. After 4s orbitals are occupied, electrons must enter 3d state (then 3d is lower in ...
- Tue Nov 17, 2020 3:54 pm
- Forum: Dipole Moments
- Topic: dipoles and polarity
- Replies: 2
- Views: 188
Re: dipoles and polarity
I am pretty sure the answer is yes. A polar molecule is a molecule whose covalently shared electrons are shared unevenly. If a molecule has a permanent dipole moment, that would mean there is a permanent difference in charge in different regions of the molecule. Lavelle points out in the lecture on ...
- Tue Nov 17, 2020 3:49 pm
- Forum: Bond Lengths & Energies
- Topic: Higher Melting Point
- Replies: 28
- Views: 2688
Re: Higher Melting Point
Comparing melting points involves looking at intermolecular attraction. To solve this you need to compare the London dispersion forces of CHI3 and CHF3. When comparing dispersion forces, CHI3's is stronger than CHF3's because the iodine in CHI3 has more electrons than the fluorine in CHF3. For dispe...
- Tue Nov 17, 2020 3:45 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Cr+ Ground State Electron Configuration
- Replies: 2
- Views: 268
Re: Cr+ Ground State Electron Configuration
I'm pretty sure that the 4s orbital has 1 electron because that is more stable that way. Lavelle explained it in the lecture on 10/26/2020.
- Tue Nov 17, 2020 3:41 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Textbook Problem 2A.3
- Replies: 1
- Views: 108
Re: Textbook Problem 2A.3
Hello.
I think that's actually problem 2A.4. 2A.3 asks for the configurations of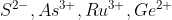
I think your answers for V4+ & Fe3+ are correct.
I think that's actually problem 2A.4. 2A.3 asks for the configurations of
I think your answers for V4+ & Fe3+ are correct.
- Fri Nov 13, 2020 10:51 pm
- Forum: Resonance Structures
- Topic: Delocalized Electrons
- Replies: 7
- Views: 372
Re: Delocalized Electrons
From my understanding, delocalized electrons are electrons not associated with single atom or covalent bond. Resonance structures represent possible structures of a molecule because there are electrons not bound to a single atom/bond.
- Fri Nov 13, 2020 10:35 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Textbook Problem 3f.5
- Replies: 2
- Views: 191
Re: Textbook Problem 3f.5
B) Butanol is more likely due to hydrogen bonding (this isn't present in diethyl ether). C) CHI3 is more likely. When comparing London dispersion forces, CHI3's is stronger than CHF3's because the iodine in CHI3 has more electrons than the fluorine in CHF3. Just to be clear, in CHF3 there is no hydr...
- Thu Nov 12, 2020 9:13 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Orbital levels
- Replies: 1
- Views: 93
Re: Orbital levels
Hello, From my understanding, the 4s orbital is lower in energy than the 3d orbital, but only slightly (in general, as energy levels increase; as they increase energies of orbitals become closer). After the 4s state is occupied, electrons must enter 3d state. Then 3d is lower in energy than 4s. When...
- Mon Nov 09, 2020 7:25 pm
- Forum: Ionic & Covalent Bonds
- Topic: # of Valence Electrons for Transition Metals [ENDORSED]
- Replies: 1
- Views: 406
# of Valence Electrons for Transition Metals [ENDORSED]
Hello.
This is a bit of a simple question but I am having some trouble understanding how to determine the number of valence electrons in transition metals?
I know that K has 1, C has 4, O has 6, etc. but what about ones like V and Ni?
This is a bit of a simple question but I am having some trouble understanding how to determine the number of valence electrons in transition metals?
I know that K has 1, C has 4, O has 6, etc. but what about ones like V and Ni?
- Mon Nov 09, 2020 7:21 pm
- Forum: Ionic & Covalent Bonds
- Topic: Textbook Question 2A.15 Part D
- Replies: 1
- Views: 127
Textbook Question 2A.15 Part D
Hello.
The question asks to write the most likely charge for the ions formed by Ga.
The answer is 3+. Why is this (my initial answer was 1+)?
My reasoning was because then there are full subshells now that there are no electrons in 4p?
The question asks to write the most likely charge for the ions formed by Ga.
The answer is 3+. Why is this (my initial answer was 1+)?
My reasoning was because then there are full subshells now that there are no electrons in 4p?
- Thu Nov 05, 2020 5:05 pm
- Forum: Student Social/Study Group
- Topic: Studying Tips
- Replies: 23
- Views: 964
Re: Studying Tips
Hello! As many have already recommended, doing the textbook problems that Lavelle has in the Outlines on his website are super helpful. I would like to add that the last problems for each section are usually more important to understand because they are harder, so if you're short on time & feel ...
- Thu Nov 05, 2020 5:01 pm
- Forum: Trends in The Periodic Table
- Topic: Midterm 2 Study Group
- Replies: 7
- Views: 477
Re: Midterm 2 Study Group
I would like to be a part of this study group :). I think this would be very helpful. How would you like me & others to contact you?
- Thu Nov 05, 2020 9:21 am
- Forum: Ionic & Covalent Bonds
- Topic: Sapling Week 5/6 #16
- Replies: 2
- Views: 127
Sapling Week 5/6 #16
Hello, I am struggling with understanding this question: A cross-link is an ionic, covalent, or hydrogen bond that links one polymer chain to another and reduces the flexibility of the polymer chains. Several possible hydrogen bond cross links are shown between the two polymer chains in the image. I...
- Thu Nov 05, 2020 8:50 am
- Forum: Ionic & Covalent Bonds
- Topic: Lengths of Single & Double Bonds
- Replies: 4
- Views: 266
Lengths of Single & Double Bonds
I don't remember if Lavelle spoke on this already, so I will just ask about it here.
If we are asked about lengths of single & double bonds on exams, do we need to memorize common ones (like C=C, or C-H) or will they be given in the question? Or do we get a reference chart for it?
If we are asked about lengths of single & double bonds on exams, do we need to memorize common ones (like C=C, or C-H) or will they be given in the question? Or do we get a reference chart for it?
- Thu Nov 05, 2020 7:36 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3695816
Re: Post All Chemistry Jokes Here
Not sure if this is chem-related but oh well.
Before heading out to the store, my sister told me "Hey, we're out of salt" to which I replied "0mg".
Before heading out to the store, my sister told me "Hey, we're out of salt" to which I replied "0mg".
- Wed Oct 28, 2020 1:34 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity
- Replies: 10
- Views: 541
Re: Electron Affinity
Hello. A higher electron affinity means an atom is more readily accepting of electrons. In terms of energy, whenever atoms gain electrons, they release energy. From my understanding, this occurs whenever electrons are gained, whether they have high or low electron affinities, but because atoms with ...
- Wed Oct 28, 2020 1:30 pm
- Forum: Properties of Light
- Topic: UA Workshop Question
- Replies: 4
- Views: 244
UA Workshop Question
Hello, I am struggling with this problem: It is generally accepted that UVC lamps with 40 mJ/cm² at a wavelength of 250 nm can be used to disinfect surfaces and kill pathogens. If you leave a UVC lamp on to disinfect a 5 m² area, how many high energy photons are produced? The answer key says 2.52*10...
- Wed Oct 28, 2020 1:21 pm
- Forum: Empirical & Molecular Formulas
- Topic: Textbook Problem M.19
- Replies: 8
- Views: 528
Re: Textbook Problem M.19
Hello. I like to write the chemical equation out like this: (?) + O2 -> CO2 + H2O + N2 I just write caffeine as (?) because we don't know its molecular formula You need to use the masses of CO2, H20, & N2 to find the moles of C, H, & N (using stoichiometry). Use the masses of C, H, & N (...
- Wed Oct 28, 2020 9:43 am
- Forum: Balancing Chemical Reactions
- Topic: Problem for UA Workshop 1
- Replies: 2
- Views: 356
Problem for UA Workshop 1
Hello. I've had a bit of trouble balancing this one chemical equation: NaHCO_3 + C_6H_8O_7\rightarrow Na_3C_6H_5O_7+H_2O+CO_2 Can anyone help me? Also does anyone have tips about how to balance equations like this? I don't want to spend too much time on these questions on the midterm (I either take ...
- Wed Oct 28, 2020 9:34 am
- Forum: Molarity, Solutions, Dilutions
- Topic: UA Session Question
- Replies: 2
- Views: 265
Re: UA Session Question
Hello.
For part B you need to use the molarity from part A & use this formula:

M1=Molarity from Part A
V1= You are solving for this.
M2=5.60*10^-7
V2=0.235L
I did the conversion, but this formula needs to be in liters, so once you find the value of V1, convert back to mL.
For part B you need to use the molarity from part A & use this formula:
M1=Molarity from Part A
V1= You are solving for this.
M2=5.60*10^-7
V2=0.235L
I did the conversion, but this formula needs to be in liters, so once you find the value of V1, convert back to mL.
- Wed Oct 21, 2020 2:27 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Homework problem #8
- Replies: 3
- Views: 152
Re: Homework problem #8
The Lyman Series all contain the transition n=2 to n=1 and Balmer Series all contain the transition n=3 to n=2 From my understanding, these are the "common transitions" because they are the biggest energy gaps in the series (the energy levels converge as they increase). In all honesty, I d...
- Wed Oct 21, 2020 2:21 pm
- Forum: Einstein Equation
- Topic: 1B.9 (7th edition)
- Replies: 8
- Views: 841
Re: 1B.9 (7th edition)
ohhh what okay then my textbook is messed up that makes so much more sense!!! Mine looks like this "1B.9 A lamp rated at emits violet light of wavelength 420 nm. How many photons of violet light can the lamp generate in 2.0 s? How many moles of photons are emitted in that time interval?" ...
- Wed Oct 21, 2020 2:14 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Variables in Rydberg Equation
- Replies: 5
- Views: 220
Re: Variables in Rydberg Equation
Frequency is not the same thing as energy level.
When you are solving the problem, you solve for the frequency and use the frequency to find the energy level. This equation involves both frequency and energy level:
)
When you are solving the problem, you solve for the frequency and use the frequency to find the energy level. This equation involves both frequency and energy level:
- Wed Oct 21, 2020 2:11 pm
- Forum: Einstein Equation
- Topic: 1B.9 (7th edition)
- Replies: 8
- Views: 841
Re: 1B.9 (7th edition)
Where does the 32W come from in this problem?? I'm confused? The 32W is given in the problem: 1B.9 A lamp rated at 32W(1W=1J⋅s^−1) emits violet light of wavelength 420 nm. How many photons of violet light can the lamp generate in 2.0 s? How many moles of photons are emitted in that time interval? o...
- Wed Oct 21, 2020 2:04 pm
- Forum: Einstein Equation
- Topic: 1B.9 (7th edition)
- Replies: 8
- Views: 841
Re: 1B.9 (7th edition)
Tikva Cohen 2I wrote:Where does the 32W come from in this problem?? I'm confused?
The 32W is given in the problem:
1B.9 A lamp rated at 32W(1W=1J⋅s^−1) emits violet light of wavelength 420 nm. How many photons of violet light can the lamp generate in 2.0 s? How many moles of photons are emitted in that time interval?
- Wed Oct 21, 2020 2:01 pm
- Forum: Administrative Questions and Class Announcements
- Topic: The Midterm Topics
- Replies: 5
- Views: 247
Re: The Midterm Topics
On Lavelle's CHEM14A website he posted an announcement saying: Midterm 1 covers material up to Wednesday of Week 3. This is up to Q19 in Sapling (quantum) homework. In Outline 2 below, all topics except the last 8 listed. MT 1 is to the end of 1D.2 in textbook. Quantum numbers, shape of orbitals, et...
- Wed Oct 21, 2020 1:57 pm
- Forum: Properties of Light
- Topic: Electromagnetic Sepectrum
- Replies: 3
- Views: 233
Re: Electromagnetic Sepectrum
From the practice/homework questions, I assume that we need to know wavelength boundaries for x-rays, infrared, microwaves, and others because those were asked in the homework. To memorize the general order, I use the mnemonic: Roman Men Invented Very Unusual X-Ray Guns Radio Waves, Microwaves, Infr...
- Wed Oct 14, 2020 10:04 pm
- Forum: General Science Questions
- Topic: Balancing equations
- Replies: 19
- Views: 2711
Re: Balancing equations
Hello. I would just like to say that sometimes when I am deep into balancing an equation and I get something that works, what happens is I went too far and have something like: 4C_{2}H_{6} + 14O_2 \rightarrow 8CO_2 + 12H_2O when it really should be: 2C_{2}H_{6} + 7O_2 \rightarrow 4CO_2 + 6H_2O Altho...
- Wed Oct 14, 2020 9:50 pm
- Forum: Properties of Electrons
- Topic: Atomic Spectra and Exciting Electrons
- Replies: 4
- Views: 153
Re: Atomic Spectra and Exciting Electrons
I believe he mentioned this in one of the Audio-Visual Focus-Topic Videos (the Atomic Spectra video)on his website. When he was drawing out the different energy levels, he mentioned that if the frequency didn't correspond to an energy difference (n=1 to n=2 for example), then it wouldn't be absorbed...

