Search found 118 matches
- Sat Mar 13, 2021 10:49 pm
- Forum: Student Social/Study Group
- Topic: Spring Quarter
- Replies: 60
- Views: 3290
Re: Spring Quarter
I'm taking Chem 14C next quarter :)
- Sat Mar 13, 2021 10:49 pm
- Forum: Ideal Gases
- Topic: Torr as a Unit of Pressure
- Replies: 8
- Views: 825
Re: Torr as a Unit of Pressure
The conversion from Torr to atm is very straightforward (1 atm = 760 torr = 760 mm Hg), and it is on our equation sheet. So if units of pressure are expressed in Torr in any of the questions on the exam, you can just use the conversion to convert it to atm :)
- Sat Mar 13, 2021 10:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: when to add Pt (s)
- Replies: 23
- Views: 1038
Re: when to add Pt (s)
You add Pt(s) when there's no conducting metal present on the cathode/anode part of a cell. For example, when both oxidized and reduced species are in aqueous state or when a solid is a not conducting, such as I2(s).
- Sat Mar 13, 2021 10:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Value of N
- Replies: 24
- Views: 1172
Re: Value of N
Hi! n is the number of moles of electrons transferred in a balanced redox reaction. Hope this helps :)
- Sat Mar 13, 2021 10:29 pm
- Forum: Calculating Work of Expansion
- Topic: W= -PDeltaV
- Replies: 15
- Views: 1928
Re: W= -PDeltaV
We use this equation when the pressure is kept constant, and there is a volume change.
- Sun Mar 07, 2021 4:30 pm
- Forum: First Order Reactions
- Topic: Slope
- Replies: 24
- Views: 930
Re: Slope
The slope is equal to negative k for zero and first order reactions, and it is equal to positive k for second order reactions.
- Sun Mar 07, 2021 4:20 pm
- Forum: Balancing Redox Reactions
- Topic: Redox Reactions
- Replies: 6
- Views: 472
Re: Redox Reactions
Adding on to what has been said, Dr. Lavelle also has 2 PDF files on his website that explain how to balance redox reactions in acidic and basic conditions. They are listed under "Redox Reactions" closer to the bottom, and I will also link them below. Balancing Redox Reactions: Acidic Cond...
- Sun Mar 07, 2021 4:12 pm
- Forum: General Rate Laws
- Topic: Rate Constant
- Replies: 31
- Views: 1229
Re: Rate Constant
Temperature and the activation energy can alter a reaction's rate constant. So if you change the temperature or add / remove a catalyst (which would change the activation energy of the reaction), it would also change the rate constant for that reaction.
- Sun Mar 07, 2021 4:02 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated Systems
- Replies: 15
- Views: 862
Re: Isolated Systems
I think that a perfectly isolated system is more of a theoretical idea that we can't completely execute in real life. We can get close to it, and a bomb calorimeter would be an example of that, but I believe that Dr. Lavelle mentioned in the lecture that, as time goes on, a bomb calorimeter becomes ...
- Sun Mar 07, 2021 3:56 pm
- Forum: First Order Reactions
- Topic: 1st Order Reactions
- Replies: 29
- Views: 1956
Re: 1st Order Reactions
Hi, So both graphs would indicate that it is a first order reaction. However, the difference is what you plot on the axes. If you plot concentration of the reactant (y-axis) vs time (x-axis) and get a decreasing exponential graph, it is a first order reaction. If you plot natural log of the concentr...
- Sun Feb 28, 2021 2:36 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Sapling #15
- Replies: 5
- Views: 360
Re: Sapling #15
Yes, since  (and remember that solids are excluded from the equilibrium constant expression), so Q=0.768/0.0150.
(and remember that solids are excluded from the equilibrium constant expression), so Q=0.768/0.0150.
- Sun Feb 28, 2021 2:14 am
- Forum: Balancing Redox Reactions
- Topic: Oxidizing Vs Reducing agent
- Replies: 39
- Views: 2081
Re: Oxidizing Vs Reducing agent
What ___ agent does:
- an oxidizing agent helps another molecule to be oxidized
- a reducing agent helps another molecule to be reduced
What happens to ___ agent itself:
- an oxidizing agent is reduced
- a reducing agent is oxidized
- an oxidizing agent helps another molecule to be oxidized
- a reducing agent helps another molecule to be reduced
What happens to ___ agent itself:
- an oxidizing agent is reduced
- a reducing agent is oxidized
- Sun Feb 28, 2021 1:55 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Which Nesnst Equation
- Replies: 25
- Views: 1192
Re: Which Nesnst Equation
If the temperature is not equal to 25 degrees Celsius, you would have to use E = E* - RT/nF(lnQ), because the shortcut form doesn't have a temperature term, which means that it can be only used when temperature is 25 degrees Celsius. However, I believe in cases when the temperature is 25 degrees Cel...
- Sun Feb 28, 2021 1:49 am
- Forum: Balancing Redox Reactions
- Topic: Determining which molecule is the oxidizing agent
- Replies: 49
- Views: 1922
Re: Determining which molecule is the oxidizing agent
Yes, you're correct! An oxidizing agent is a molecule that is reduced and that is helping another molecule to be oxidized. Conversely, a reducing agent is a molecule that is oxidized and that is helping another molecule to be reduced.
- Sun Feb 28, 2021 1:30 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: When to use Different Nernst Equations
- Replies: 11
- Views: 904
Re: When to use Different Nernst Equations
I believe that the main difference is that the second form of the Nernst equation can be only used when temperature is 25 degrees Celsius (298.15K), since there is no term for temperature in the equation; in cases when the temperature is 25 degrees Celsius, the equations can be used interchangeably....
- Sun Feb 21, 2021 2:13 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G and G naught
- Replies: 46
- Views: 4606
Re: Delta G and G naught
The main difference between delta G and delta G naught is that delta G naught is delta G at standard conditions (1atm and 25 degrees Celsius / 298.15 K).
- Sun Feb 21, 2021 2:10 pm
- Forum: Van't Hoff Equation
- Topic: Vant Hoff's equation
- Replies: 7
- Views: 510
Re: Vant Hoff's equation
To calculate K at a different temperature using Van't Hoff equation, you need to know the value of K at some other temperature as well as the delta H for that reaction.
- Sun Feb 21, 2021 2:07 pm
- Forum: Van't Hoff Equation
- Topic: Application of Van't Hoff Equation
- Replies: 11
- Views: 3879
Re: Application of Van't Hoff Equation
We can use Van't Hoff equation to find the value of the equilibrium constant at a different temperature, given that we know the enthalpy of the reaction and the equilibrium constant at some other temperature. We can also use it to find the enthalpy of the reaction, given that we know values of equil...
- Sun Feb 21, 2021 2:04 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: State function?
- Replies: 36
- Views: 1398
Re: State function?
No, E (cell potential) is not a state function, which means that we cannot subtract the final state from initial to find the desired value, like we've done for enthalpy, entropy and Gibbs Free Energy.
- Sun Feb 21, 2021 2:00 pm
- Forum: Calculating Work of Expansion
- Topic: Work sign
- Replies: 46
- Views: 2078
Re: Work sign
Hi, When work is done by the system, work is a negative value. The way you can think of this is that when work is done by the system, it means that the system is dong work and, in doing so, it's losing energy. Therefore, work is negative. When work is done on the system, work is a positive value. Ag...
- Sun Feb 14, 2021 6:04 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed vs Isolated System
- Replies: 30
- Views: 1363
Re: Closed vs Isolated System
In both closed and isolated systems, matter cannot be exchanged with the surroundings. However, in a closed system, energy can be exchanged with the surroundings whereas, in an isolated system, it cannot. An example of a closed system is a cup of coffee with a lid on it or a closed plastic water bot...
- Sun Feb 14, 2021 5:58 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy: kJ or J?
- Replies: 30
- Views: 1608
Re: Entropy: kJ or J?
I think units for entropy are usually J/K but you may have to convert it to kJ to perform certain calculations or if the question asks you to give your answer in kJ, so it's best to always look at what the problem gives you and what it is asking for. Hope this helps :)
- Sun Feb 14, 2021 5:43 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Adding Equations
- Replies: 16
- Views: 811
Re: Adding Equations
Yes, as others have stated, since delta S and delta G are both state properties, just like delta H is, we can apply the same rules, which are: - adding their values when adding the equations - multiplying the value of delta H/S/G by a coefficient if you multiply the equation associated with that val...
- Sun Feb 14, 2021 5:33 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: when K=1
- Replies: 12
- Views: 519
Re: when K=1
It is uncommon for K value to be equal 1, because it means that neither reactants nor products are favored at equilibrium. Thus, it follows that since K=1 is uncommon, delta G = 0 would be uncommon as well.
- Sun Feb 14, 2021 5:29 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Sapling Number 5
- Replies: 8
- Views: 475
Re: Sapling Number 5
First, you need to find the number of moles, using PV=nRT. Since the pressure is given in kPa, we can use the following value for the gas constant: R = 8.314 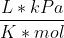 .
.
Then, you'll use) to find the change in entropy.
to find the change in entropy.
Then, you'll use
- Fri Feb 05, 2021 1:36 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Constant pressure/ volume
- Replies: 11
- Views: 488
Re: Constant pressure/ volume
Hi! No, one variable being constant does not mean that others are constant as well. So constant temperature does not imply that pressure is constant, and constant pressure does not imply that pressure is constant. If we look at the ideal gas law, PV=nRT, we can see the direct relationship between pr...
- Fri Feb 05, 2021 1:29 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy Question
- Replies: 8
- Views: 336
Re: Internal Energy Question
Since the change in internal energy is the sum of heat and work, whether it is a positive or a negative value depends on the values for heat and work.
- Fri Feb 05, 2021 1:27 pm
- Forum: Ideal Gases
- Topic: Gas Constant Value
- Replies: 43
- Views: 1664
Re: Gas Constant Value
Since there are several different values for the gas constant that differ, depending on the units, look at the units you are dealing with in the problem and choose the appropriate R value (that is, make sure that units match up). The best way to double check whether you chose the correct value for R...
- Fri Feb 05, 2021 1:19 pm
- Forum: Calculating Work of Expansion
- Topic: Work notation
- Replies: 10
- Views: 456
Re: Work notation
Hi! Work is denoted by lowercase w :)
- Fri Feb 05, 2021 1:19 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy definition
- Replies: 37
- Views: 2532
Re: Entropy definition
Entropy is a measure of disorder or chaos of a system. In chemistry, it typically refers to molecules, so the more spread out the molecules are, the larger the entropy of a system.
- Fri Feb 05, 2021 1:16 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Adiabatic system
- Replies: 3
- Views: 229
Re: Adiabatic system
Hi! Yes, you're correct. An adiabatic process occur without the transfer of heat between the system and its surroundings. This also means that the energy is transferred to the surroundings only as work. Hope this helps :)
- Sun Jan 31, 2021 5:20 pm
- Forum: Phase Changes & Related Calculations
- Topic: Define Phase Change
- Replies: 78
- Views: 5307
Re: Define Phase Change
The official definition of phase change is as follows: a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. So, essentially, a phase change occurs whenever matter goes from one state to another. There is a diagram ( http://www.chemistry.wustl.edu/~edudev/L...
- Sun Jan 31, 2021 5:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: midterm
- Replies: 16
- Views: 609
Re: midterm
Based on 14A last quarter, it took around 1-1.5 weeks for our scores to be released. If there are no mistakes on the test that would need to be fixed to make sure everyone's tests are graded fairly, I'd say we can expect our scores to come out towards the end of Week 5 (so a week after we took the m...
- Sun Jan 31, 2021 5:05 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: polyprotic acids
- Replies: 10
- Views: 675
Re: polyprotic acids
Adding on to what has been said above, according to the textbook, we can ignore second deprotonation for all polyprotic acids we will encounter in this class, except for H2SO4. While it is still good to compare Ka1 and Ka2 values to double check that we can ignore second deprotonation, I think what ...
- Sun Jan 31, 2021 4:58 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: State Function
- Replies: 22
- Views: 781
Re: State Function
Work is not a state function because it depends on the path taken to get from initial state to the final. More specifically, it is due to the fact that work is proportional to the distance an object is moved.
- Sun Jan 31, 2021 4:49 pm
- Forum: Student Social/Study Group
- Topic: Midterm Scores
- Replies: 25
- Views: 887
Re: Midterm Scores
Hi! Based on last quarter, it usually took around 1-1.5 weeks. If there are no mistakes in the test that would require additional time to verify that everyone's scores were graded fairly, I'd say we can expect results to come out in about a week from the day of the exam (so towards the end of Week 5...
- Sun Jan 31, 2021 4:45 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14C?
- Replies: 23
- Views: 974
Re: 14C?
Hi! Splitting 14C and 14D from spring to fall quarters is actually a really good question to pose that I didn't think about before. I feel like it's just a matter of personal preference as well as what classes you're planning to take in the spring / back-up options you have. As of right now (althoug...
- Sat Jan 23, 2021 4:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 #7
- Replies: 3
- Views: 250
Re: Sapling Week 2 #7
Hi! Your value for Kb is incorrect. Remember that Kw = Ka * Kb, so to find Kb from Ka, you need to do the following: Kb = Kw / Ka = 10^-14 / Ka. In this case, Kb should be equal to 2.5*10^-7. The rest of your set up and solving process seems to be correct, so I think you just need to follow the same...
- Sat Jan 23, 2021 4:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: X Approximations
- Replies: 23
- Views: 909
Re: X Approximations
You can assume x value is small enough to approximate it when the equilibrium constant (whether it is Ka, Kb, etc) is smaller than or equal to 10^{-4} . You can also double check whether using the approximation in a given case was valid by using the 5% rule. If % ionization or protonation is less th...
- Sat Jan 23, 2021 4:41 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Weak Acids
- Replies: 8
- Views: 403
Re: Weak Acids
Hi! The general equation for we use for weak acids is: HA_{(aq)} + H_{2}O_{(l)} \rightleftharpoons H_{3}O_{(aq)}^{+} + A_{(aq)}^{-} And the general equation we use for weak bases is: B_{(aq)} + H_{2}O_{(l)} \rightleftharpoons HB_{(aq)}^{+} + OH...
- Sat Jan 23, 2021 3:56 pm
- Forum: Student Social/Study Group
- Topic: Midterm
- Replies: 21
- Views: 887
Re: Midterm
The midterm will be 50 minutes long, and I believe we will be given 5 additional minutes as a buffer in case there are any problems with the program or submission (at least that's how it was last quarter for 14A). The number of questions as well as the amount of points each question is worth change ...
- Sat Jan 23, 2021 3:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: H3O+ and OH- concentration
- Replies: 3
- Views: 151
Re: H3O+ and OH- concentration
Hi! I believe you're correct that we can assume that [H3O+] = [OH-] in that case even if the Kw is not equal to 10^-14 due to a change in temperature. To confirm your assumption, you could set up an ICE table for this reaction, and you will get the same value for [H3O+] and [OH-]. However, I'm not s...
- Sun Jan 17, 2021 3:37 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Using the ICE table
- Replies: 36
- Views: 1424
Re: Using the ICE table
Hi! Yes, you can use the ICE table when you're given Kp and partial pressures as well as when you're given Kc and molar concentrations. Just make sure that your values are consistent throughout your ICE table (meaning that all of your values given in either partial pressures or molar concentrations,...
- Sun Jan 17, 2021 3:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Chart with Gas Pressures
- Replies: 8
- Views: 327
Re: ICE Chart with Gas Pressures
Hi! Yes, you're correct! If we're given Kp and partial pressures, we can use ICE table just like we would if we were given Kc and molar concentrations. And since 1 atm = 1.01325 bar, Dr. Lavelle mentioned in his lecture that, in this course, we can approximate that 1 atm = 1 bar to simplify our appr...
- Sun Jan 17, 2021 3:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc and Kp
- Replies: 11
- Views: 468
Re: Kc and Kp
Hi! The way I think about it is that Kc is a more general one because it can be applied to both aqueous solutions and gases whereas Kp only applies to gases. So you can choose either Kc or Kp for gases, depending whether you're given partial pressures of gases (then you use Kp) or molar concentratio...
- Sun Jan 17, 2021 3:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterms
- Replies: 24
- Views: 1062
Re: Midterms
Hi! Midterms will take place during your Friday lecture on weeks 4 and 7.
Here's the link to the Exam Schedule posted on Dr. Lavelle's website that outlines that: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Chem14BTestSchedule.pdf
Here's the link to the Exam Schedule posted on Dr. Lavelle's website that outlines that: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Chem14BTestSchedule.pdf
- Sun Jan 17, 2021 3:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Posting on Chem Community
- Replies: 6
- Views: 356
Re: Posting on Chem Community
I was also wondering what happens if someone originally posts under the wrong topic, and we reply to their post? In that case, would our posts count towards the 5 posts needed for the week or not?
- Sun Jan 17, 2021 2:58 pm
- Forum: Ideal Gases
- Topic: Bars to atm [ENDORSED]
- Replies: 41
- Views: 1884
Re: Bars to atm [ENDORSED]
Hi!
So 1 atm = 1.01325 bar, but since the values are so close, Dr. Lavelle mentioned in his lecture that, in this course, we will just assume that 1 atm = 1 bar to simplify our calculations. Hope this helps :)
So 1 atm = 1.01325 bar, but since the values are so close, Dr. Lavelle mentioned in his lecture that, in this course, we will just assume that 1 atm = 1 bar to simplify our calculations. Hope this helps :)
- Sun Jan 10, 2021 3:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Question about 14B Exams
- Replies: 38
- Views: 1421
Re: Question about 14B Exams
Since midterms will be administered during class time, do you think we will still have a lecture to watch on Fridays of week 4 and week 7?
- Sun Jan 10, 2021 3:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units
- Replies: 27
- Views: 965
Re: Units
I think for this course, we'll mostly use atm as units for pressure, but since 1 bar is approximately equal to 1 atm, we'll use bar as well.
- Sun Jan 10, 2021 3:03 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Clarification of terminology
- Replies: 15
- Views: 672
Re: Clarification of terminology
Yes, all of the three phrases express the same idea, which is that more products will be formed. And, as Dr. Lavelle mentioned in the lecture, there may be some confusion with the term "shift," because "shift" typically means "change," but K value is not changing. For t...
- Sun Jan 10, 2021 2:45 pm
- Forum: Student Social/Study Group
- Topic: Advice for someone who didn't take 14A with professor Lavelle
- Replies: 61
- Views: 2956
Re: Advice for someone who didn't take 14A with professor Lavelle
Hi! I also found UA sessions to be extremely helpful last quarter. I'd suggest going to many different UAs in the first couple weeks to see whose style / worksheets you like and then stick going to those UAs for the rest of the quarter. In addition, I try to read the textbook and take notes on it (w...
- Sun Jan 10, 2021 2:34 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Changing volume
- Replies: 6
- Views: 355
Re: Changing volume
You can generalize the "Quick Way" shown in the lecture to any reaction as the following: - when volume is increased, the side with more moles of gas will be favored - when volume is decreased, the side with less moles of gas will be favored This way, reactions adjust to minimize the effec...
- Fri Jan 08, 2021 7:48 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature Change
- Replies: 4
- Views: 261
Re: Temperature Change
The way I memorized it was:
- Endothermic: as T increases, K increases (up up)
- Exothermic: as T increases, K decreases (up down)
- Endothermic: as T increases, K increases (up up)
- Exothermic: as T increases, K decreases (up down)
- Thu Dec 10, 2020 12:23 am
- Forum: Bronsted Acids & Bases
- Topic: Textbook 6C.19 part f
- Replies: 2
- Views: 220
Re: Textbook 6C.19 part f
In both molecules, the hydrogen atom is bonded to oxygen, so O-H bond is the same length. In that case, we don't look at size of the atom and rather figure out the anion of which acid is more stable since the more stable the anion, the stronger the acid. Since both molecules have equal number of oxy...
- Thu Dec 10, 2020 12:12 am
- Forum: Polyprotic Acids & Bases
- Topic: Proton vs H+
- Replies: 14
- Views: 793
Re: Proton vs H+
Yes, they are used interchangeably. It is because 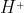 has no electrons and only 1 proton.
has no electrons and only 1 proton.
- Thu Dec 10, 2020 12:05 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration of Scandium
- Replies: 4
- Views: 816
Re: Electron Configuration of Scandium
In one of the UA sessions, it was explained that, at first, 4s has lower energy, which is why it gets filled up first. However, once electrons occupy the 3d orbital, the energy "settles down," and 3d now has lower energy that 4s, so we write 3d and then 4s.
- Wed Dec 09, 2020 11:53 pm
- Forum: Student Social/Study Group
- Topic: Review Session
- Replies: 11
- Views: 653
Re: Review Session
Since Dr. Lavelle finished covering the material today (Wednesday), the review on Friday will be during our "lecture" time, so it will be recorded and posted on CCLE.
- Wed Dec 09, 2020 11:50 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Why does nickel have an expanded octet?
- Replies: 6
- Views: 744
Re: Why does nickel have an expanded octet?
Ni is in period 4 and is a transition metal, so it has 3d orbitals that it can use to have expanded octet.
Also, Ni does not have f-orbitals. Remember that f-orbitals lag by 2, so 4f orbitals do not appear until period 6.
Hope this helps :)
Also, Ni does not have f-orbitals. Remember that f-orbitals lag by 2, so 4f orbitals do not appear until period 6.
Hope this helps :)
- Mon Dec 07, 2020 10:58 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Preference on Scientific Notation
- Replies: 13
- Views: 763
Re: Preference on Scientific Notation
Both are correct, but I'd stick to the 1.0 x10^6 one as opposed to the shorthand one because 1.0 x10^6 is more commonly used, and 1.0 E6 is more associated with calculations on a calculator and such. However, I don't think it will matter for this class because our final, just like our midterms, is m...
- Fri Dec 04, 2020 7:04 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Date
- Replies: 21
- Views: 1166
Re: Final Exam Date
Our final is on December 13th.
You can find this information on his website, it's under "Test and Exam Schedule," and here's the link: https://lavelle.chem.ucla.edu/wp-conten ... hedule.pdf
You can find this information on his website, it's under "Test and Exam Schedule," and here's the link: https://lavelle.chem.ucla.edu/wp-conten ... hedule.pdf
- Fri Dec 04, 2020 6:53 pm
- Forum: General Science Questions
- Topic: Tricks for Knowing Locations of Metals and Nonmetals on Periodic Table
- Replies: 8
- Views: 5782
Re: Tricks for Knowing Locations of Metals and Nonmetals on Periodic Table
I think memorizing where metalloids are located is the most important because if you know where metalloids are, you also know that metals are to the left of it and nonmetals are to the right. The way I memorized the location of metalloids on the periodic table is that I imagine a mental staircase th...
- Fri Dec 04, 2020 6:42 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591882
Re: Post All Chemistry Jokes Here
A little throwback to when classes were in-person and we did labs :"


- Fri Dec 04, 2020 6:31 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Equilibrium sign
- Replies: 9
- Views: 903
Re: Equilibrium sign
No, we don't write the equilibrium sign when writing reactions for the dissociation of a strong acid/strong base because the assume that they dissociate completely.
- Fri Dec 04, 2020 6:26 pm
- Forum: Hybridization
- Topic: Sigma/pi bonds
- Replies: 6
- Views: 178
Re: Sigma/pi bonds
Hi! No, the order doesn't matter. And the questions usually simply ask us to list how many sigma and pi bonds are there :)
- Fri Dec 04, 2020 5:50 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Molecular Geometry vs. Electron Geometry
- Replies: 6
- Views: 342
Re: Molecular Geometry vs. Electron Geometry
You are correct that you only consider the bonds when determining the molecular geometry. And the shape of the molecule is just another name for molecular geometry. As for electron geometry, Dr. Lavelle often referred to it as "the arrangement of electron density," but they both mean the s...
- Wed Nov 25, 2020 6:57 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: H2O VSEPR
- Replies: 27
- Views: 1310
Re: H2O VSEPR
Hi! It's important to differentiate between the arrangement of electron densities and the shape of a molecule.
H2O has 4 regions of electron densities. Therefore, it has tetrahedral arrangement of electron densities.
However, because 2 of them are lone pairs, the shape of the molecule is bent.
H2O has 4 regions of electron densities. Therefore, it has tetrahedral arrangement of electron densities.
However, because 2 of them are lone pairs, the shape of the molecule is bent.
- Wed Nov 25, 2020 6:43 pm
- Forum: Hybridization
- Topic: Sapling Week 7 & 8 HW Question 11
- Replies: 14
- Views: 828
Re: Sapling Week 7 & 8 HW Question 11
How I remembered it is you just count the "number of things" attached to the central atom, where single/double/triple bonds & lone pairs count as a single region on electron density (so "1 thing"). P has 3 single bonds & 1 lone pair, so it has 4 things attached. Therefore...
- Wed Nov 25, 2020 6:35 pm
- Forum: Sigma & Pi Bonds
- Topic: delocalized pi bonds and resonance clarification
- Replies: 3
- Views: 228
Re: delocalized pi bonds and resonance clarification
Yes, you're correct. Because when there are only single bonds (and, therefore, only sigma bonds), there can't be resonance since all bonds are the same. Thus, resonance can only exist when a molecule has at least one double bond (for example, CO_{3}^{2-} ), and, as we learned, a double bond consists...
- Wed Nov 25, 2020 6:28 pm
- Forum: Hybridization
- Topic: Hybridization with double bonds
- Replies: 4
- Views: 203
Re: Hybridization with double bonds
Hi! Single/double/triple bonds & lone pairs all count as a single region on electron density.
Thus, a molecule with 2 single bonds and a double bond would have sp^2 hybridization because the "number of things" attached to the central atom is 3.
Thus, a molecule with 2 single bonds and a double bond would have sp^2 hybridization because the "number of things" attached to the central atom is 3.
- Wed Nov 25, 2020 6:22 pm
- Forum: Hybridization
- Topic: Long Pairs/Double & Triple Bonds
- Replies: 9
- Views: 514
Re: Long Pairs/Double & Triple Bonds
Hi! An easy way to remember this is: when determining hybridization, count everything that's attached to the atom.
Thus, this includes single/double/triple bonds & lone pairs, and they all count as a single region of electron density.
Thus, this includes single/double/triple bonds & lone pairs, and they all count as a single region of electron density.
- Mon Nov 23, 2020 12:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Radicals and Biradicals in VSEPR Model
- Replies: 4
- Views: 229
Re: Radicals and Biradicals in VSEPR Model
Yes, Dr. Lavelle mentioned in one of the lectures that single electrons are also counted as a single region of electron density, along with lone pairs of electrons & bonding pairs.
- Sat Nov 21, 2020 7:05 pm
- Forum: Student Social/Study Group
- Topic: Finals!
- Replies: 43
- Views: 1957
Re: Finals!
Hi! Yes, I believe the final is cumulative :)
- Sat Nov 21, 2020 7:01 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Strongest/weakest intermolecular forces
- Replies: 6
- Views: 428
Re: Strongest/weakest intermolecular forces
Here's the list of intermolecular forces in the order of increasing strength (from weakest to strongest):
- LDF (induced dipole-induced dipole)
- dipole-induced dipole
- dipole-dipole
- ion-dipole
- hydrogen bonding
- ion-ion
- LDF (induced dipole-induced dipole)
- dipole-induced dipole
- dipole-dipole
- ion-dipole
- hydrogen bonding
- ion-ion
- Fri Nov 20, 2020 1:10 am
- Forum: Dipole Moments
- Topic: Midterm 2 Results
- Replies: 7
- Views: 440
Re: Midterm 2 Results
I think it should be released some time next week, like it was with MT1, or possibly sooner since there is no partial credit this time.
- Fri Nov 20, 2020 1:06 am
- Forum: Bond Lengths & Energies
- Topic: Bond Strength in DNA
- Replies: 12
- Views: 905
Re: Bond Strength in DNA
I'm not sure by how much but G-C pair is definitely stronger since, as you mentioned, G-C pair can form 3 hydrogen bonds whereas A-T pair can form only 2. In his lectures, Dr. Lavelle mentioned that the effect of IMFs adds up, and, thus, G-C pairs are more stable.
- Fri Nov 20, 2020 12:59 am
- Forum: Bond Lengths & Energies
- Topic: Boiling vs. Melting Point
- Replies: 15
- Views: 2812
Re: Boiling vs. Melting Point
The boiling point is the temperature at which a liquid evaporates and turns into gas whereas the melting point is the temperature at which a solid melts to become a liquid. For example, at the melting point of water, ice turns into liquid water; and at its boiling point, water evaporates and turns i...
- Thu Nov 19, 2020 12:20 am
- Forum: Octet Exceptions
- Topic: Aluminum bonding
- Replies: 5
- Views: 385
Re: Aluminum bonding
Similar to boron, aluminum typically forms 3 bonds. For example, AlCl3.
- Sat Nov 14, 2020 10:36 pm
- Forum: Dipole Moments
- Topic: Hydrocarbons
- Replies: 3
- Views: 190
Re: Hydrocarbons
It doesn't matter which name you use since all of them (induced dipole-induced dipole, LDF, Van der Waals) are used interchangeably. Hope this helps :)
- Sat Nov 14, 2020 10:29 pm
- Forum: Dipole Moments
- Topic: Sapling number 17
- Replies: 10
- Views: 487
Re: Sapling number 17
My question didn't have CH4 as one of the choices, but CH4 only exhibits LDF because it is nonpolar due to its molecular shape. When you draw the Lewis structure of CH4, you can see that all C-H bonds will cancel each other out and, thus, the molecule as a whole will be nonpolar.
- Sat Nov 14, 2020 10:23 pm
- Forum: Lewis Structures
- Topic: dipole dipole intermolecular forces
- Replies: 4
- Views: 272
Re: dipole dipole intermolecular forces
The strength of dipole-dipole forces depends on the molecule's polarity. Thus, as the more polar the molecule is, the stronger the dipole-dipole interactions it forms.
- Sat Nov 14, 2020 10:14 pm
- Forum: Lewis Structures
- Topic: Sapling #4
- Replies: 8
- Views: 280
Re: Sapling #4
Hi! Both of your Lewis structures are correct, except you incorrectly identified formal charges on the second one (where there is a double bond between C and N). N does have a positive formal charge. However, the formal charge on C is 0, and the formal change on both O is -1.
- Sat Nov 14, 2020 10:10 pm
- Forum: Lewis Structures
- Topic: Sapling HW week 5/6 question 13
- Replies: 3
- Views: 218
Re: Sapling HW week 5/6 question 13
For this question, you need to identify the number of sites where there could be a potential hydrogen bond. Thus, you need to count N/O/F with a lone pair (note that if an atom has 2 lone pairs, it counts as 2 hydrogen bond sites) and number of H atoms bonded to N/O/F. For example, on the left side,...
- Thu Nov 12, 2020 6:26 pm
- Forum: Lewis Structures
- Topic: Lewis Structures for Large Molecules
- Replies: 4
- Views: 358
Re: Lewis Structures for Large Molecules
If the molecule you need to draw a Lewis structure for is a hydrocarbon/similar organic compound, write down a chain of C atoms first. From there, you know that each carbon can form up to 4 bonds and that C-H bonds are singular bonds and C-O bonds are double bonds. Based on this information, put dow...
- Fri Nov 06, 2020 6:30 pm
- Forum: Resonance Structures
- Topic: Bond lengths
- Replies: 20
- Views: 750
Re: Bond lengths
We don't have to draw double / triple bonds shorter than single bonds when drawing Lewis structures. Lewis structures are just a way to represent what molecule structure looks like, so they don't have to drawn to scale. Just know that the bond length decreases as the number of bonds increases :)
- Fri Nov 06, 2020 6:21 pm
- Forum: Electronegativity
- Topic: Electron Affinity
- Replies: 6
- Views: 165
Re: Electron Affinity
While noble gases are very stable due to them having a complete octet and don't form compounds under ordinary conditions, some heavier noble gases will form compounds with highly reactive ions (ex. halogens) when ionized and under pressure. It is due to the fact that heavier noble gases have more el...
- Fri Nov 06, 2020 6:11 pm
- Forum: Resonance Structures
- Topic: Resonance Structures and Bond Lengths
- Replies: 3
- Views: 385
Re: Resonance Structures and Bond Lengths
I think the best you can do when it comes to determining a bond length in a resonance structure is to approximate that it would be the average of all bonds. For example, if there are 2 single and 1 double bonds in a resonance structure, the length of bonds would be the sum of the three bonds divided...
- Fri Nov 06, 2020 6:00 pm
- Forum: Student Social/Study Group
- Topic: Blind sided by Midterm 1 memorization questions, How to study for memorization questions
- Replies: 11
- Views: 388
Re: Blind sided by Midterm 1 memorization questions, How to study for memorization questions
Writing things down helps me to remember the information better, so I try to take thorough notes on the text from the textbook (and I also took notes on the videos from the modules). I highlighted and looked over these notes a couple days and then a day before the midterm. To be honest, taking notes...
- Fri Nov 06, 2020 5:53 pm
- Forum: Lewis Structures
- Topic: Difference in ionic and covalent Lewis structures
- Replies: 9
- Views: 2778
Re: Difference in ionic and covalent Lewis structures
Since covalent compounds share electrons, we draw a line between the 2 atoms indicating that. However, in an ionic compound, that doesn't happen, and one atom donates its electron to another, more electronegative atom. As a result, we don't draw a line between them but rather use brackets ([]) to si...
- Thu Nov 05, 2020 9:33 am
- Forum: Ionic & Covalent Bonds
- Topic: Lengths of Single & Double Bonds
- Replies: 4
- Views: 265
Re: Lengths of Single & Double Bonds
I don't think we need to memorize them, so I believe if we would need them to solve a problem, they'd be given in the question. However, it's useful to know the general correlation that as the number of bonds increases, the length of the bonds decreases. For example, a double bond is shorter than a ...
- Fri Oct 30, 2020 11:26 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Textbook Question 1B.27
- Replies: 3
- Views: 136
Re: Textbook Question 1B.27
Hi! It is a typo in the answer key. So you're correct, 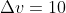 m/s. You can find the correct solution in Solution Manual Errors (https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf), which is also posted on Dr. Lavelle's website. Hope this helps :)
m/s. You can find the correct solution in Solution Manual Errors (https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf), which is also posted on Dr. Lavelle's website. Hope this helps :)
- Fri Oct 30, 2020 10:54 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Quick Question about Wave Functions and their Definition
- Replies: 3
- Views: 216
Re: Quick Question about Wave Functions and their Definition
Yes, you're correct. So a wave function ( \psi _{n, l, m_{l}} ) that is a solution with quantum numbers n, l, m_{l} is an orbital, and wave function squared is different from that; \psi ^{2} is probability density, which means that the probability of finding particle in a region is proportional to t...
- Fri Oct 30, 2020 10:49 am
- Forum: Properties of Light
- Topic: Sapling Question 24
- Replies: 4
- Views: 129
Re: Sapling Question 24
Hi! There are 2 things you need to look at to determine whether a wave is compatible or not. First, if you look at the given options, you'll notice that some of the waves are irregular, meaning that they have different frequencies and amplitudes within one wave. Irregular waves are not compatible. S...
- Thu Oct 29, 2020 10:27 am
- Forum: Administrative Questions and Class Announcements
- Topic: Audio-Visual Focus Topics
- Replies: 14
- Views: 485
Re: Audio-Visual Focus Topics
I found them very helpful as well! However, I believe Dr. Lavelle mentioned that they take a long time to make, so while he might create more in the coming years, it's highly unlikely that more modules would be added this quarter.
- Thu Oct 29, 2020 9:59 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg's
- Replies: 7
- Views: 326
Re: Rydberg's
Rydberg's equation is a modified version of 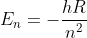 , which comes empirical observations rather that being derived, so it only works for H-atoms. Therefore, Rydberg's equation also works only for H-atoms.
, which comes empirical observations rather that being derived, so it only works for H-atoms. Therefore, Rydberg's equation also works only for H-atoms.
- Thu Oct 29, 2020 9:52 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: position units
- Replies: 19
- Views: 1384
Re: position units
I think it's best to convert it to meters, so it is consistent with units of velocity (m/s) and momentum (kg*m/s) and you don't make mistakes when calculating the final answer. Then, if the problem asks you to give your final answer in nm, just convert it. Hope it helps :)
- Sun Oct 25, 2020 5:13 pm
- Forum: Einstein Equation
- Topic: Textbook Problem 1A.3
- Replies: 9
- Views: 481
Re: Textbook Problem 1A.3
Choice A isn't the right answer because the speed of radiation is always constant and equal to the speed of light (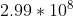 m/s). Therefore, as frequency decreases, the wavelength increases, so that their product would always be equal to the speed of light. Hope this helps :)
m/s). Therefore, as frequency decreases, the wavelength increases, so that their product would always be equal to the speed of light. Hope this helps :)
- Sun Oct 25, 2020 10:58 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Lyman and Balmer Series
- Replies: 5
- Views: 406
Re: Lyman and Balmer Series
Hi! Lyman and Balmer series are the only ones that have been emphasized in this course so far, and I haven't seen any problems that would require you to know the other series. So I believe knowing these two should be enough. However, if you want to know the third series as well, and it's called Pasc...
- Sun Oct 25, 2020 10:51 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: P-, d-, f- Orbitals
- Replies: 5
- Views: 325
Re: P-, d-, f- Orbitals
You are correct. A nodal plane is a region where there is zero probability of finding an electron in that area. Thus, the fact that p-, d- and f-orbitals have nodal planes means that there are areas in those orbitals (the area is the nodal plane) where the likelihood of finding electrons is zero.
- Sun Oct 25, 2020 10:43 am
- Forum: DeBroglie Equation
- Topic: Derivation of Equations on Midterm?
- Replies: 17
- Views: 587
Re: Derivation of Equations on Midterm?
Hi! Since the midterm is all multiple choice, I don't think we'll have to necessarily show our work / how we derived equations. However, since Dr. Lavelle went over the derivation of De Broglie equation, and it is also one of the bullet points on the "Quantum World" outline, I believe we d...

