Search found 101 matches
- Sun Mar 07, 2021 10:25 pm
- Forum: Student Social/Study Group
- Topic: Note Taking
- Replies: 145
- Views: 19543
Re: Note Taking
I personally handwrite everything since it does sort of force me to think about what I'm writing. Also taking the time to maybe pause lecture and rephrase his slides in a way that I understand/shorter than what Lavelle wrote also helps me with learning the material. I also reinforce the lecture note...
- Sun Mar 07, 2021 10:20 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagram orders
- Replies: 4
- Views: 280
Re: Cell diagram orders
To add onto the post above, if you want to be super meticulous about the order (like Sapling #7 from last week I think?) place the solid metals at the ends and the aqueous ions that interact with salt bridge in the middle (surrounding the double line ||). But I personally don't think it matters that...
- Sun Mar 07, 2021 10:15 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Solving Multiple Reactant Problems
- Replies: 3
- Views: 278
Re: Solving Multiple Reactant Problems
Unless I'm severely misunderstanding what we're learning (which is honestly completely possible), I believe you're right in saying the total rate is equal to the slowest elementary step. The reaction can only proceed as fast as the rate-determining step (slowest step) which is why the rate law uses ...
- Sun Mar 07, 2021 10:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 7
- Views: 490
Re: Cell Diagrams
I believe that order does matter (at least it does in Sapling #7 from last week). I think that the aqueous ions should be on the inner end:
Pt(s) / Cl2 (g) / Cl- (aq) // K+ (aq)/ K2 (g) / Pt(s)
Sapling specified that the ions that make contact with salt bridge should be placed in the middle.
Pt(s) / Cl2 (g) / Cl- (aq) // K+ (aq)/ K2 (g) / Pt(s)
Sapling specified that the ions that make contact with salt bridge should be placed in the middle.
- Sun Mar 07, 2021 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Making X Negligible
- Replies: 6
- Views: 685
Re: Making X Negligible
We can't make X negligible because X has a significant effect on K when it's greater than 10^-4. We say X is negligible when K is less than 10^-4 because it essentially has no effect on the denominator of the fraction. For instance (X^2)/(3-X). As K becomes smaller, ignoring K on the bottom matters ...
- Sun Mar 07, 2021 12:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Combining K's
- Replies: 7
- Views: 500
Re: Combining K's
Remember that K is equal to the [products]/[reactants] of a chemical reaction at a given temperature. When you add two chemical reactions, you have to find K again with the "new" products and reactants. As for why we cant make K negative, K is a ratio of products to reactants. It wouldn't ...
- Sun Mar 07, 2021 12:33 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: What is the purpose of having a salt bridge?
- Replies: 16
- Views: 808
Re: What is the purpose of having a salt bridge?
The salt bridge allows for ion transfer (and subsequently electron transfer). Without the salt bridge, the electrons moving from the anode to the cathode would build up and electron flow would become unfavorable causing the reaction to stop. The salt bridge moves spectator ions between the two solut...
- Sun Mar 07, 2021 12:23 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 21212
Re: How do you deal with burnout?
I was feeling the burnout a second ago. Personally, I need a lot of rest and just separation from school for a few hours, maybe a day. At these times, the most I do is look at emails to make sure I'm not missing some deadline but aside from that school mentally doesn't exist. To get back into school...
- Sun Mar 07, 2021 12:17 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Calculating K from Ecell
- Replies: 2
- Views: 228
Re: Calculating K from Ecell
I obviously can't speak for Lavelle but from past answer choices, I think there's usually a clear difference in answer choices. He also usually gives specific values that we should use in order to avoid rounding differences. In the example you gave, he would probably ask something like "report ...
- Sun Mar 07, 2021 12:10 pm
- Forum: Balancing Redox Reactions
- Topic: What is the tip to balance basic and acidic redox reactions with the x and H/OH and H20?
- Replies: 2
- Views: 266
Re: What is the tip to balance basic and acidic redox reactions with the x and H/OH and H20?
This might be what you're thinking of? "If you need x more O atoms on one side, you should add 2x OH− ions to that side, then x H2O molecules to the opposite side."
- Sat Feb 27, 2021 10:38 pm
- Forum: Balancing Redox Reactions
- Topic: PSA on Sapling #5 for week 7/8
- Replies: 3
- Views: 222
Re: PSA on Sapling #5 for week 7/8
Just to add to this, Sapling really did not like it when I put OH- in the middle of the Pb(OH)4 equation. Not sure how if the order of other compounds affects it but if anyone else is having trouble (and the issue isn't with the coefficients), try putting OH- at the end.
- Sat Feb 27, 2021 10:32 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling #5
- Replies: 2
- Views: 227
Re: Sapling #5
It's a bit hard to see the problem without your work but since you said you have extra electrons, I'm assuming that you already balanced the equation for a basic solution? If you haven't, you need to add H2O and OH- to the magnesium oxide half-rxn and balance that. You may also be getting extra elec...
- Sat Feb 27, 2021 10:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling week 7/8 #13
- Replies: 2
- Views: 626
Re: Sapling week 7/8 #13
You're on the right track with having to compare the standard reduction potentials of the given reagents with the answer choices. To oxidize one reaction without oxidizing the other would mean that the standard reduction potential of your answer should be in between the standard reduction potentials...
- Sat Feb 27, 2021 10:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Hydrogen Electrode
- Replies: 3
- Views: 240
Re: Standard Hydrogen Electrode
It's designed to have 0V as the E naught so that we can calculate cell potentials with varying electrodes and concentrations without having to factor in the E naught of the SHE.
- Sat Feb 27, 2021 10:06 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling wk 7/8 #5
- Replies: 6
- Views: 398
Re: Sapling wk 7/8 #5
dana hu 1B wrote:Thank you for your response
I then got this which is also wrong:
2MnO4-(aq0 + 4H2O(l) + 3S+2(aq) -> 2MnO2(s) +3S(s) +8OH-(g)
What did I do wrong here? Are my states wrong?
Yup, OH- should be aqueous, your charge on S should be 2- too
- Sun Feb 21, 2021 2:39 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Non-spontaneous exothermic reaction
- Replies: 14
- Views: 4632
Re: Non-spontaneous exothermic reaction
Spontaneous processes are when ∆G = ∆H-T∆S is negative. In an exothermic process, ∆H is negative but this doesn't always mean ∆G is also negative. If ∆S is negative and the temperature is high enough, ∆G can become positive. I think this is called an entropy-driven reaction or entropy is dominant. I...
- Sun Feb 21, 2021 2:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5J.15
- Replies: 3
- Views: 232
Re: 5J.15
Another way to approach this using the same deltaG = deltaH - TdeltaS equation is to only find the deltaS of the formation reaction by using the standard molar entropies of formation. Since a lower deltaG is more stable, we need to see the sign of the deltaS. If deltaS is positive, increasing the te...
- Sun Feb 21, 2021 2:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D. 9
- Replies: 2
- Views: 398
Re: 4D. 9
I had a lot of problems with this exercise too. To find the energy released, you're supposed to find the enthalpy of the reaction using the standard enthalpies of formation. This gives us the amount of energy released in the total reaction but since we need energy per mole of TNT, we need to divide ...
- Sun Feb 21, 2021 2:18 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calibrating Calorimeter
- Replies: 6
- Views: 2872
Re: Calibrating Calorimeter
Calibrating a calorimeter means measuring a calorimeter's heat capacity by carrying out a reaction that releases a known quantity of heat and then observing the change in temperature. We can then use this heat capacity in future procedures/calculations that use the same calorimeter.
- Thu Feb 18, 2021 8:49 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook 4D.7
- Replies: 4
- Views: 323
Textbook 4D.7
Oxygen difluoride is a colorless, very poisonous gas that reacts rapidly and exothermically with water vapor to produce O2 and HF: OF2(g) + H2O(g) → O2(g) + 2HF(g). ΔH=-318 kJ. What is the change in internal energy for the reaction of 1.00 mol of OF2? I understand that we have to use the delta U= de...
- Sun Feb 14, 2021 9:33 pm
- Forum: Student Social/Study Group
- Topic: Chem 14BL
- Replies: 10
- Views: 901
Re: Chem 14BL
If you really want an in-person lab experience, you're better off waiting. But if you're fine with having simulated labs/just watching labs and having the data given to you, then I'd recommend taking 14BL. I'm taking it with Casey so I can't really speak for any other professors but the class is ver...
- Sun Feb 14, 2021 9:21 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: sapling #6
- Replies: 1
- Views: 178
Re: sapling #6
I could be wrong but I thought you only need the variations of R when computing the Cv,m or Cp,m of the gas. Since the equation of delta S for a change in Volume only states R, we don't need to use the 5R/2 because then you would be inputting the Cv,m of Neon into the equation, not the gas constant.
- Sun Feb 14, 2021 9:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: What are the three ways to find Delta H?
- Replies: 7
- Views: 12912
Re: What are the three ways to find Delta H?
Hi! I remember Dr. Lavelle stating that some of these are more reliable than the others, and I was wondering which ones are more accurate and why. Thank you! Hess' Law and Standard Enthalpies of Formation are about the same level of reliability and are the more reliable ones. These are specific to ...
- Sun Feb 14, 2021 9:00 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Sapling question 7
- Replies: 8
- Views: 489
Re: Sapling question 7
I misread this at first but it's actually because the question is asking for orderliness when the samples are liquids. Larger ΔSvap means that when a liquid became gas, the entropy changed by the ΔSvap. Since C2H5OH has the largest ΔSvap, it's gas form is the most disorderly but it's liquid form is ...
- Sun Feb 14, 2021 8:48 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: standard change in enthalpy vs. change in enthalpy
- Replies: 4
- Views: 308
Re: standard change in enthalpy vs. change in enthalpy
Standard change in enthalpy and change in enthalpy are both in terms of energy so they would both use Joules. Standard change in enthalpy is commonly reported as kJ/mol and is used under standard conditions (298K and 1atm). Change in enthalpy is usually reported as kJ or J (doesn't really matter) an...
- Sun Feb 07, 2021 9:41 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy, definition and explaination
- Replies: 3
- Views: 121
Re: Entropy, definition and explaination
The textbook definition of entropy is "(1) A measure of the disorder of a system. (2) A change in entropy is equal to the heat supplied reversibly to a system divided by the temperature at which the transfer takes place." It's a bit of a weird concept but it's sort of a measure of how mess...
- Sun Feb 07, 2021 9:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Week 3/4 sampling #6
- Replies: 3
- Views: 200
Re: Week 3/4 sampling #6
When you see a chemical reaction that involves O2 in the reactants and CO2 and H2O in the products, it's a combustion reaction. So it would be the enthalpy of combustion. But if you forgot that, you can also look at the +/- 4×bond energy of C−H and notice that the reaction involves the breaking of O...
- Sun Feb 07, 2021 9:29 pm
- Forum: Student Social/Study Group
- Topic: Best kdrama?
- Replies: 30
- Views: 2208
Re: Best kdrama?
I'm actually not too familiar with most kdrama but I got into watching "Run On" (on Netflix) and I'm surprised no one recommended it yet. It's fairly new and the season finale came out not too long ago. It's a romcom so take that as you will lol.
- Sun Feb 07, 2021 9:24 pm
- Forum: Calculating Work of Expansion
- Topic: Sapling #14
- Replies: 11
- Views: 548
Re: Sapling #14
I used the PV=nRT equation, substituting the P, T, and V with the initial conditions of the problem
- Sun Feb 07, 2021 9:22 pm
- Forum: Calculating Work of Expansion
- Topic: Work Equations
- Replies: 3
- Views: 262
Re: Work Equations
I don't think there's one that's necessarily preferred over another, like you said, it depends on what information is given in the problem. The context of the problem and what information they provide will ultimately determine which equation to use. The actual equation is -\int_{v1}^{v2}P_e_x dV but...
- Sun Jan 31, 2021 10:24 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: percent protonation/deprotonation
- Replies: 15
- Views: 931
Re: percent protonation/deprotonation
They're basically the same thing but deprotonation is used for acids while protonation is used for bases. If you recall, H3O+ is sometimes substituted with H+ (or a proton). Since acids give away protons when dissociated, we use %deprotonation with them. Meanwhile bases accept protons so we use %pro...
- Sun Jan 31, 2021 10:19 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure affects only gas reactions?
- Replies: 23
- Views: 1947
Re: Pressure affects only gas reactions?
Yup, since solids and liquids basically don't change concentration ever, while concentrations of aqueous things only change when solvent or solute is added, pressure will only affect the gases. Gases fill up the container they're in so if they're compressed, their concentrations change. If there's e...
- Sun Jan 31, 2021 10:11 pm
- Forum: Student Social/Study Group
- Topic: 14C and CL
- Replies: 8
- Views: 795
Re: 14C and CL
I haven't taken either so I'm really just reciting what I've heard from my upperclassman so take it with a grain of salt. It's definitely possible to take C and CL concurrently since they cover the material again in lab but if you feel like you need a more in-depth background with the topics, most p...
- Sun Jan 31, 2021 10:02 pm
- Forum: Calculating Work of Expansion
- Topic: assuming room temperature
- Replies: 4
- Views: 502
Re: assuming room temperature
Yup, it's common practice for books to forego telling the temperature since it's usually assumed that it's room temperature unless specified otherwise. If there's no way to solve for the temperature with the given information, I'd say to either consider if temperature is even relevant to the problem...
- Sun Jan 31, 2021 9:56 pm
- Forum: Calculating Work of Expansion
- Topic: Gas Constant
- Replies: 13
- Views: 871
Re: Gas Constant
Look at the units of your temperature, pressure, energy, and moles. The units of your R constant should be the same so that they cancel out when used. You may have to do some dimensional analysis to get the right value of R.
- Sun Jan 24, 2021 7:53 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam
- Replies: 33
- Views: 2096
Re: Steam
It's because steam is a gaseous state. If you remember the phase diagram Lavelle showed, there's a significant amount of energy needed to convert water into steam. When steam hits your skin, it condenses back to water and all that energy released from changing from a gas back into a liquid is releas...
- Sun Jan 24, 2021 7:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Quadratics when assumption can't be used
- Replies: 5
- Views: 282
Re: Quadratics when assumption can't be used
Sorry if this is a vague answer but it's a bit hard to discern what the problem is since your numbers may vary from mine and I can't see your work but I suggest to make sure the values you input are correct, you're using the right units, and to double-check your signs when you did the algebra to wri...
- Sun Jan 24, 2021 7:41 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reversible Reaction
- Replies: 5
- Views: 292
Re: Reversible Reaction
Like the person above said, since enthalpy is the change in energy between the products and reactants, the one-way arrow is probably used to denote that the given change in enthalpy specifically applies to the forward reaction. I think the reverse reaction's change in enthalpy would just be the nega...
- Sun Jan 24, 2021 7:37 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Changes
- Replies: 4
- Views: 278
Re: State Changes
My interpretation of phase changes are a bit different than work functions for electron ejection. In the photoelectric effect, as long as the minimum energy was met the electron was ejected. Phase changes are similar in that there is a specific amount of energy between phases that have to be overcom...
- Sun Jan 24, 2021 7:25 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: pH of weak acid
- Replies: 9
- Views: 454
Re: pH of weak acid
Depends on how accurate you want to be but a general rule of thumb is when Ka is less than 10^-4 or 10^-5 (Sapling sometimes says when the acid concentration is 1000 times greater than the Ka). But always make sure to check that the x-value you find is less than 5% of the original concentration othe...
- Fri Jan 15, 2021 9:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When x is negligible in Equilibrium constant
- Replies: 34
- Views: 1704
Re: When x is negligible in Equilibrium constant
As others have said K should be less than 10^-3 but to using 10^-4 is safer. If that's hard to remember, Sapling says that if the molarity of the initial concentration is 1000 times greater than the K, you can assume x is negligent.
- Fri Jan 15, 2021 9:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant
- Replies: 2
- Views: 190
Re: Equilibrium Constant
According to the lecture from last Friday, only temperature can change the equilibrium constant. So if the temperature remains constant, I don't think starting with a higher pressure will result in a larger K value.
- Fri Jan 15, 2021 9:41 pm
- Forum: Ideal Gases
- Topic: Gas for equilibrium equation
- Replies: 8
- Views: 473
Re: Gas for equilibrium equation
I think we have to use P before a molecule to denote that we're using the partial pressure of that molecule, otherwise, it may be misconstrued as the concentration.
- Fri Jan 15, 2021 9:38 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box
- Replies: 28
- Views: 1396
Re: ICE Box
I'd say unless you know for sure that you're working with a strong acid/base (i.e. stated in the problem) to just use the ICE box. It'd be a lot more efficient to only use the OH-/H3O+ concentrations when you know you're working with strong acids/bases but if you're unsure, using the ICE box would b...
- Fri Jan 15, 2021 9:30 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: sapling Q.5
- Replies: 2
- Views: 96
Re: sapling Q.5
I could be wrong but the line after you found the [OH-] concentration, I think you miswrote the exponent on the Kb as -3 instead of -5
- Sun Jan 10, 2021 8:48 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure and Volume
- Replies: 16
- Views: 861
Re: Pressure and Volume
They're inversely related. At constant temperature, increasing one will cause the other to decrease. Vice versa, decreasing one will increase the other. As for why, increasing pressure is the same as compressing a system (which effectively decreases the volume). Or when volume is increased, there is...
- Sun Jan 10, 2021 8:45 pm
- Forum: Administrative Questions and Class Announcements
- Topic: missing section
- Replies: 7
- Views: 518
Re: missing section
Sections aren't mandatory so missing one is fine. If you're concerned about what you missed, you can email your TA/go to their office hours about what they went over during discussion. You can also attend the UA sessions to catch up on what you missed.
- Sun Jan 10, 2021 8:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and pressure
- Replies: 5
- Views: 350
Re: K and pressure
Temperature is the only condition that can change K since it involves a change in the energy of the system which affects whether the forward or reverse reactions will be favored. However, like the others previously stated, changing pressure is equivalent to changing the volume and thus changes the c...
- Sun Jan 10, 2021 8:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: identifying solids and non homogeneous reactant/product
- Replies: 4
- Views: 291
Re: identifying solids and non homogeneous reactant/product
Unless the reaction is a well-known precipitation reaction (e.g. NaCl(aq) + AgNO3(aq) ----> AgCl(s) + NaNO3) which is a reaction that always results in an insoluble solid, then we may be expected to know that a substance is a solid. But as far as I know, we haven't been taught that yet so I'm inclin...
- Sun Jan 10, 2021 8:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Conditions of Equilibrium
- Replies: 4
- Views: 206
Re: Conditions of Equilibrium
I'm a little confused with your question so I might give unnecessary info but the conditions of equilibrium are usually given to us in the problem (with the context of problems that we've been introduced to so far). Future problems may require us to solve for certain conditions given other condition...
- Fri Dec 11, 2020 5:12 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 10
- Views: 798
Re: hybridization
When we draw the lewis structure of NH3, we get 3 N-H bonds with one lone pair on the N. Which means that N has 4 regions of electron density and since hybridization depends on the # of electron density regions, we also need 4 hybridized orbital which correlates to a sp3 hybridization.
- Fri Dec 11, 2020 5:06 pm
- Forum: Sigma & Pi Bonds
- Topic: Benzene Sigma/Pi Bonds
- Replies: 3
- Views: 360
Re: Benzene Sigma/Pi Bonds
Remember that each double bond is composed of a sigma bond and a pi bond. With that in mind, there are 6 sigma bonds from each of the single C-C bonds and another 6 sigma bonds from each of the C-H bonds. The 3 pi bonds comes from the alternating double C=C bonds in the carbon ring.
- Fri Dec 11, 2020 4:59 pm
- Forum: Polyprotic Acids & Bases
- Topic: Textbook 6C.19
- Replies: 7
- Views: 532
Re: Textbook 6C.19
Relative strength of an acid is related to it's ability to lose an H+ ion. So acids where it's very easy to lose a proton are relatively stronger than acids where it is harder to lose an proton. In other words, weaker bonds=stronger acid. With HF and HCl, the bond between HF is very short because of...
- Fri Dec 11, 2020 4:26 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Why are spectator ions of a salt related to strong acids/bases?
- Replies: 2
- Views: 282
Why are spectator ions of a salt related to strong acids/bases?
I understand why ions of weak acids and bases can make the solutions more alkaline/acidic when interacting with water but I don't get why ions of strong acids/bases don't? What is the reason these strong acid/base ions don't act like the ions of weak acids/bases?
- Fri Dec 11, 2020 4:10 pm
- Forum: Lewis Acids & Bases
- Topic: Week 10 Sapling #6
- Replies: 7
- Views: 645
Re: Week 10 Sapling #6
The way the book defines weak acid/base is any acid/base classified as strong. https://sites.google.com/site/chempendix/strong-acids-bases . So any acid/base not listed here is considered weak. Since none of the ones you still have left are on the strong acid/base list they are all weak/other NaCl: ...
- Sat Dec 05, 2020 8:47 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Quick rundown on coordination numbers?
- Replies: 4
- Views: 196
Quick rundown on coordination numbers?
I don't know why but this just isn't clicking with me. Is the coordination number just the amount of bonds/ligands to the central cation? And what relationship do dentates have with the CN (if any)?
- Sat Dec 05, 2020 8:32 pm
- Forum: Lewis Structures
- Topic: Lone Pair placement
- Replies: 10
- Views: 695
Re: Lone Pair placement
The position of lone pairs is dependent on the electron geometry and is determined by what is the most favorable position (usually opposite from each other and equatorial). H2O has tetrahedral geometry and two lone pairs. It's a bit hard to envision but if you look it up, it really doesn't matter wh...
- Sat Dec 05, 2020 8:20 pm
- Forum: Lewis Acids & Bases
- Topic: Bronsted vs. Lewis bases
- Replies: 11
- Views: 438
Re: Bronsted vs. Lewis bases
I think so. Bronsted bases are proton acceptors while Lewis bases are electron donors, so while all Bronsted bases are also Lewis bases, I believe not all Lewis bases are Bronsted bases. For instance BF3 + F- ----> BF4-. F- is considered the Lewis base since it donated its lone pair but in and of it...
- Sat Dec 05, 2020 8:05 pm
- Forum: Bronsted Acids & Bases
- Topic: Do acids always list hydrogens as the first element in a molecule?
- Replies: 3
- Views: 188
Re: Do acids always list hydrogens as the first element in a molecule?
As the person said above, I'd say it depends on whether we're discussing Lewis acids or Bronsted acids. Lewis acids are defined as electron pair acceptors, so compounds like BF3 which accepts a lone pair from F-, is considered an acid but doesn't contain any H. However, Bronsted acids (which the tex...
- Sat Dec 05, 2020 7:56 pm
- Forum: Hybridization
- Topic: Unhybridized orbitals
- Replies: 5
- Views: 300
Re: Unhybridized orbitals
Since the number of electron density regions is equivalent to the amount of hybridized orbitals, whatever's left is unhybridized. For instance, in a compound there's 2 e- density regions which means there's 2 hybridized orbitals, or a sp hybridization. Since the p subshell has 3 orbitals but only 1 ...
- Sun Nov 29, 2020 9:07 pm
- Forum: Hybridization
- Topic: How to find the hybridization
- Replies: 14
- Views: 786
Re: How to find the hybridization
This is the way I understand it so anyone is free to correct me if I'm wrong lol. The # of regions of electron density surrounding an atom is equivalent to the # of hybrid orbitals. So if an atom has 3 bonds and 1 lone pair, there should be 4 hybrid orbitals. The type of hybridization is then determ...
- Sun Nov 29, 2020 8:50 pm
- Forum: Electronegativity
- Topic: Does electronegativity cancel?
- Replies: 4
- Views: 467
Re: Does electronegativity cancel?
From my understanding, electronegativities do not cancel. They're not like charges where formal charges of atoms within a molecule contribute to the overall charge of the molecule. Electronegativity "stays" with its atom, for lack of a better word. In a molecule where one atom is highly el...
- Sun Nov 29, 2020 8:43 pm
- Forum: Lewis Structures
- Topic: Sapling #12
- Replies: 3
- Views: 255
Re: Sapling #12
Yup! You can check by comparing the molar mass of CH4O with the given molar mass of the unknown compound since they should match. CH4O is approximately 32.041 g/mol which is essentially the same as the MM of the unknown compound so you should have the right molecular formula.
- Sun Nov 29, 2020 8:37 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: H20 Shape
- Replies: 17
- Views: 1110
Re: H20 Shape
The square planar shape requires an octohedral electron geometry where 2 electron density regions are lone pairs. Since H2O only has 4 regions of electron density, it has tetrahedral geometry and so it's not possible for it to be square planar nor linear. Now given a tetrahedral geometry with two lo...
- Sun Nov 29, 2020 8:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Resonance Structure Definition
- Replies: 9
- Views: 523
Re: Resonance Structure Definition
So in other words, its basically the exact same molecule/lewis structure except you move the double bond/triple bond to another of the same atom? Yes, to everything but that last part. The atoms are all in the same spot and only the double/triple bonds are "moving". They don't always have...
- Sun Nov 29, 2020 8:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: How do bonds affect shapes?
- Replies: 11
- Views: 796
Re: How do bonds affect shapes?
The type of bond (single, double, triple) does not affect the shape of the molecule, or at least it doesn't affect the overall shape of the molecule significantly. All bonds and lone pairs (LP) are treated as regions of electron density. You're somewhat on the right track with counting the amount of...
- Sun Nov 29, 2020 8:08 pm
- Forum: Lewis Structures
- Topic: Octet Exceptions
- Replies: 4
- Views: 197
Re: Octet Exceptions
I think the rule of thumb is that p-block of n=3 and below can accommodate an expanded octet because they have a d-subshell to fit more valence electrons into (although they don't always have an expanded octet). As for a set/maximum number of electrons, (I really don't know so this is really just my...
- Sat Nov 21, 2020 11:44 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Outline 2: Objective #12
- Replies: 6
- Views: 431
Re: Outline 2: Objective #12
Remember that atomic orbitals exist in 4 different shapes, or wave functions (s, p, d, f). We don't really know the exact position of an electron at any given point in time but by finding the square of the wave function, we can find the probability of finding an electron in a given orbital. It's har...
- Sat Nov 21, 2020 11:25 am
- Forum: Trends in The Periodic Table
- Topic: Book Problem 1F3
- Replies: 5
- Views: 543
Re: Book Problem 1F3
When atoms are isoelectronic (here they all have the same # of electrons as Argon), the size of the ion essentially boils down to which ion has the most protons. The more protons there are, the more positive charges there are to attract all the electrons (as someone said before this is called the ef...
- Sat Nov 21, 2020 11:20 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Textbook question 1E.9
- Replies: 4
- Views: 512
Re: Textbook question 1E.9
It'd first be helpful to remember the "bounds" of the quantum number and that the numbers are ordered by {n, l, ml, ms}. l can be 0 to (n-1), ml can be -l to +l (including 0), and ms can only be +1/2 or -1/2. Given these bounds, we can say that (a) is valid set of quantum numbers since non...
- Sat Nov 21, 2020 11:12 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook 2E #27c
- Replies: 6
- Views: 348
Re: Textbook 2E #27c
The difference in electronegativity between C and Cl would result in three of the bonds in CHCl3 to be polar. The C-H bond is also slightly polar but it's neither as strong as C-Cl or as numerous. Because of the shape of CHCl3 (tetrahedral if I'm not mistaken), then the electron density of the molec...
- Sat Nov 21, 2020 11:03 am
- Forum: Bond Lengths & Energies
- Topic: Boiling vs. Melting Point
- Replies: 15
- Views: 2960
Re: Boiling vs. Melting Point
Boiling point is the temperature where a compound changes from liquid to gas whereas the melting point is where a molecule/element changes from solid to liquid. A simple example of boiling is when water becomes water vapor, and melting is when ice becomes water. In context of what we're learning rig...
- Tue Nov 17, 2020 1:07 pm
- Forum: Lewis Structures
- Topic: Textbook Question Number 2B 11 part C
- Replies: 2
- Views: 126
Re: Textbook Question Number 2B 11 part C
Yeah so the resonance structures would be the double bond of the carbon switching between the two oxygens since both theoretically work. But as the person above said, the answer the book gave is more favorable since there are no charged atoms in the compound.
- Tue Nov 17, 2020 12:56 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: subshells and orbitals
- Replies: 9
- Views: 2235
Re: subshells and orbitals
I think you have it backward, orbitals are parts of a subshell, not the other way around. The order goes shell>subshell>orbital. Within a given subshell (s,p,d,f), there is a certain number of orbitals that can each contain 2 electrons.
- Tue Nov 17, 2020 12:49 pm
- Forum: Trends in The Periodic Table
- Topic: Periodic Trends Summarized [ENDORSED]
- Replies: 8
- Views: 1238
Re: Periodic Trends Summarized [ENDORSED]
Atomic/Ionic radius: Trend: Increases down a group and increases as you go left across a period (or you could remember it as decreases as it goes right). Increases as you approach bottom left corner Reason: bigger atoms have larger radii and larger atoms in the same period have more nuclear charge s...
- Tue Nov 17, 2020 12:18 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic and Covalent Character
- Replies: 2
- Views: 153
Re: Ionic and Covalent Character
I think the trend would still apply. Remember that ionic character and covalent character aren't mutually exclusive. Even in Groups 1/2, the size of the atoms increases which makes it easier for electrons to be shared since they're farther away from the nucleus and which then increases covalent char...
- Tue Nov 17, 2020 12:12 pm
- Forum: Lewis Structures
- Topic: Lewis acids and Bases
- Replies: 20
- Views: 938
Re: Lewis acids and Bases
Since Lewis acids are electron acceptors and Lewis bases are electron donors, it's best to draw the Lewis dot structure of a compound to see their valence electrons. Compounds that are electron-deficient and need electrons for an octet such as BF3 are bases, while compounds that have lone-pair elect...
- Sat Nov 07, 2020 7:17 pm
- Forum: Properties of Electrons
- Topic: Elements with similar number of electrons
- Replies: 9
- Views: 399
Re: Elements with similar number of electrons
I'm not sure what your question is really saying since you said similar number of electrons. Since this could technically refer to isoelectronic atoms (different elements that have the same amount of electrons) and elements that are close together on the periodic table (close number of electrons at ...
- Sat Nov 07, 2020 7:09 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Angular momentum quantum #s over 3
- Replies: 4
- Views: 143
Re: Angular momentum quantum #s over 3
Theoretically, there would be a g, h, etc. subshells but due to the Aufbau principle, an atom would need A LOT of electrons to fill all the way to the g-subshell and beyond (no ground state element has enough, as far as I know). So for practical purposes, the s, p, d, f-subshells are the only ones w...
- Sat Nov 07, 2020 7:02 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Isoelectric atoms
- Replies: 9
- Views: 717
Re: Isoelectric atoms
Isoelectronic atoms have the same number of electrons. Their charges would differ depending on how many electrons were added/removed to result in the atoms to be comparatively isoelectronic. Electronegativity and ionization energies are also different despite the same amount of electrons since these...
- Sat Nov 07, 2020 6:56 pm
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 10
- Views: 473
Re: Resonance Structures
Resonance structures are the individual structures of a particular molecule. In a sense, it's each possible drawing where the bond(s) is/are shifting. The resonance hybrid is the mix of all these resonance structures. Like in the case of benzene, the resonance structures are the two possible hexagon...
- Sat Nov 07, 2020 6:52 pm
- Forum: Electronegativity
- Topic: Fluorine
- Replies: 7
- Views: 395
Re: Fluorine
Firstly, since Fluorine already has 7 valence electrons, it really "wants" another valence electron to fulfill the octet rule. This is the case for all the other halogens as well. Now why it's more electronegative compared to the other halogens, as the others have said, is due to Fluorine'...
- Sun Nov 01, 2020 12:32 pm
- Forum: Ionic & Covalent Bonds
- Topic: Strength of bonds
- Replies: 13
- Views: 679
Re: Strength of bonds
From highest to lowest I believe the bond strength (in general) is as follows: multiple covalent bonds > single covalent bonds > ionic bonds > hydrogen bonds > van der Waals force.
- Sun Nov 01, 2020 12:22 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: when n=5 and l=4
- Replies: 16
- Views: 4636
Re: when n=5 and l=4
When the quantum number n=5, l can theoretically equal 4 but is not necessarily (or realistically 4). When n=5, l can be 0,1,2,3,4 since the values of l range from 0 to n-1. l=4 would also result in subshell g but as far as I know, no ground state elements have electrons in the g subshell.
- Sun Nov 01, 2020 12:17 pm
- Forum: Lewis Structures
- Topic: Octet Rule
- Replies: 12
- Views: 525
Re: Octet Rule
H, He, Li, and Be don't apply to the octet rule since they essentially only have access to the 1s subshell which will be stable with (and can only contain) two electrons. But other exceptions exist such as boron and aluminum that often don't follow the octet rule but Lavelle hasn't covered those yet...
- Sun Nov 01, 2020 12:11 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Unusual Electron Configuration
- Replies: 3
- Views: 765
Re: Unusual Electron Configuration
It's a bit of a difficult concept and I'm not too sure if I have a complete understanding as well but I think the gist of it is that filling the electrons using the Aufbau principle does not always result in the most stable atom. In the case of copper, the config is [Ar]4s^2 3d^9 . I believe the sta...
- Sun Nov 01, 2020 11:58 am
- Forum: Lewis Structures
- Topic: Dot order
- Replies: 11
- Views: 900
Re: Dot order
It really doesn't matter where the electrons are located or how they are filled. Though many use the start at the top and fill in a clockwise way to fill Lewis structures, at the end of the day, I think the dot orders are just a visual interpretation designed to easily see the valence e- of atoms/co...
- Sun Nov 01, 2020 11:53 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: N levels for electron configurations
- Replies: 6
- Views: 377
Re: N levels for electron configurations
The quantum number ℓ would specify the orbital shape aka the subshell (s, p, d, f). In this case, it would mean the p-subshell and each subshell has a specific amount of electrons (s=2 max e-, p=6 max e-, etc.)
- Tue Oct 27, 2020 10:38 pm
- Forum: Empirical & Molecular Formulas
- Topic: Book problem E15
- Replies: 2
- Views: 285
Book problem E15
E.15 The molar mass of the metal hydroxide M(OH)2 is 74.10g⋅mol−1. What is the molar mass of the sulfide of this metal? The notation kind of confused me but is it basically saying that "M" is an arbitrary element? And to solve you would just subtract the molar mass of the (OH)2 and add the...
- Fri Oct 23, 2020 5:12 pm
- Forum: Trends in The Periodic Table
- Topic: Do elements retain electronegativity even when ionized?
- Replies: 3
- Views: 203
Do elements retain electronegativity even when ionized?
This is pertaining to #16 on sapling which asks to order ions according to atomic radius. All the ions have the same amount of electrons so I'm thinking the radius has to be determined by how electronegative the element is when it's not ionized?
- Sat Oct 17, 2020 4:46 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Module Question 28
- Replies: 9
- Views: 439
Re: Photoelectric Effect Module Question 28
Right, for this specific part of the problem, the work function doesn't have any use. If I remember correctly, the work function was used in a different problem with the same context. But since you asked what else we can derive from the work function (aka threshold energy or energy to remove electro...
- Sat Oct 17, 2020 4:32 pm
- Forum: Einstein Equation
- Topic: m vs nm
- Replies: 66
- Views: 4119
Re: m vs nm
Unless specified otherwise, both units would be acceptable since m is the SI unit for length and nm is the common unit for wavelength. But if you want to be safe, you could also just write both units since it's a fairly quick and easy conversion.
- Sat Oct 17, 2020 4:26 pm
- Forum: Student Social/Study Group
- Topic: How are you studying?
- Replies: 204
- Views: 25857
Re: How are you studying?
I agree with everyone's studying methods above but honestly what works for other people isn't always gonna work for you. So definitely try out everyone's suggestions but also figure out what helps you learn best. For me, I'm also not particularly good at staying on top of work if I don't designate s...
- Sat Oct 17, 2020 4:17 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Module Question 28
- Replies: 9
- Views: 439
Re: Photoelectric Effect Module Question 28
Hi! You're right that m would be the mass of the electron but keep the units as kilograms (9.109 × 10^-31 kg). Not only are kilograms the SI unit for mass but since energy in Joules is equivalent to 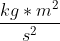 , keeping the mass in kilograms is necessary to get the correct answer.
, keeping the mass in kilograms is necessary to get the correct answer.
- Sat Oct 17, 2020 4:11 pm
- Forum: Properties of Light
- Topic: Speed of Light Equation
- Replies: 6
- Views: 314
Re: Speed of Light Equation
I believe the speed of light and it's related equations should remain constant in a vacuum but does change when travelling through a medium (like air, water, etc.). For our class, I believe we'll only work with the assumption that light is travelling in a vacuum and in any non-vacuum cases, he will ...
- Sat Oct 17, 2020 4:03 pm
- Forum: Properties of Light
- Topic: "Intensity"
- Replies: 20
- Views: 803
Re: "Intensity"
I'm essentially reiterating everyone's answers but yes they are the same. When referring to intensity of a light, we're talking about the amount of photons that are being emitted by a particular light source. Each individual photon has the same amplitude but since there's so much of them, they add u...
- Thu Oct 15, 2020 4:20 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: WK 2 Sapling #5
- Replies: 4
- Views: 292
WK 2 Sapling #5
Could someone explain how to find the number of spectral lines when an electron drops from a specific energy level? I'm not sure how to approach this problem. "The electron in a hydrogen atom is excited to energy level n=6 and emits electromagnetic radiation when returning to lower energy level...
- Tue Oct 13, 2020 5:45 pm
- Forum: Photoelectric Effect
- Topic: Textbook Question 1B15
- Replies: 4
- Views: 203
Re: Textbook Question 1B15
Hi!
Yeah, you do have to convert km/s to m/s since all the units need to be in SI units.
You would also need to use the de Broglie equation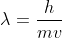 where m is the mass of the electron (9.1 x 10^-31 kg).
where m is the mass of the electron (9.1 x 10^-31 kg).
Yeah, you do have to convert km/s to m/s since all the units need to be in SI units.
You would also need to use the de Broglie equation
- Thu Oct 08, 2020 5:04 pm
- Forum: SI Units, Unit Conversions
- Topic: Sig figs and molar mass
- Replies: 18
- Views: 668
Re: Sig figs and molar mass
Like everyone else said, it's typically best to use the listed molar mass on the periodic table. Second best option would probably be to round to at least 3 decimals. It's always best to get the most accurate answer we can (but if you are in a timecrunch, rounding elements like hydrogen, carbon, and...
- Thu Oct 08, 2020 4:50 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect question help
- Replies: 1
- Views: 93
Re: Photoelectric Effect question help
Hi! To start off, since the question is asking for kinetic energy were gonna have to use the equation Ek = \frac{1}{2}mv^{2} where m and v are the mass and velocity of the electron (in this part of the question, the work function doesn't have any purpose). Inputting the mass of an electron (approx 9...

