Search found 104 matches
- Sat Mar 06, 2021 9:36 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity
- Replies: 10
- Views: 469
Re: Molecularity
Molecularity just shows us how many molecules are participating in the rate determining step of a mechanism.
- Sat Mar 06, 2021 9:32 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Week 10 Review Sessions
- Replies: 10
- Views: 561
Re: Week 10 Review Sessions
I highly recommend Rosa and Wesley. They've been super helpful for 14A & 14B.
- Sat Mar 06, 2021 9:31 pm
- Forum: Balancing Redox Reactions
- Topic: How to Determine if a Reaction is in Basic or Acidic Solution?
- Replies: 53
- Views: 3260
Re: How to Determine if a Reaction is in Basic or Acidic Solution?
Since the textbook and sapling problems tell you what type of solution it is in, Professor Lavelle will likely tell us on a final question.
- Sat Mar 06, 2021 9:30 pm
- Forum: First Order Reactions
- Topic: 1st Order Reactions
- Replies: 29
- Views: 1979
Re: 1st Order Reactions
A straight line represents the order based on one of the three formulas. A first order reaction is a straight line when graphing ln[A], a second order reaction is a straight line when graphing 1/[A], and a zero order reaction is a straight line when graphing [A].
- Sat Mar 06, 2021 9:25 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DAYLIGHT SAVINGS TIME!
- Replies: 12
- Views: 1440
Re: DAYLIGHT SAVINGS TIME!
Thank you for the reminder! This is really important not to forget.
- Sat Mar 06, 2021 9:24 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 11408
Re: What was your favorite chem topic?
I really enjoyed the quantum unit!
- Sat Mar 06, 2021 9:21 pm
- Forum: Student Social/Study Group
- Topic: What do you miss / What are you looking forward to?
- Replies: 92
- Views: 10325
Re: What do you miss / What are you looking forward to?
I'm looking forward to meeting new people and making friends! Hopefully I will be able to get involved with in-person research as well.
- Sat Mar 06, 2021 9:19 pm
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 375966
Re: Final Jitters
I always make sure to get enough sleep two nights before the exam. Usually I plan my studying so that I do not need to cram the information the day before the test. Right before the exam, I wash my face with cold water and take a nice deep breath.
- Sat Mar 06, 2021 9:17 pm
- Forum: General Science Questions
- Topic: Midterm 2 Reactions
- Replies: 79
- Views: 6546
Re: Midterm 2 Reactions
I was surprised at how many conceptual problems there were on midterm 2. Although I didn't do as well as I hoped, the test was definitely fair. Hopefully we will finish the new content soon so that we will have more time to review for the final!
- Sat Mar 06, 2021 9:14 pm
- Forum: Student Social/Study Group
- Topic: Chem 14C
- Replies: 17
- Views: 965
Re: Chem 14C
I've heard that 14C isn't as bad as people make it out to be. My friend told me its a lot of memorization, but not very difficult conceptually.
- Sat Mar 06, 2021 9:06 pm
- Forum: Student Social/Study Group
- Topic: 14B Final
- Replies: 22
- Views: 1067
Re: 14B Final
It is highly likely the final will be divided equally. He will probably send an email soon telling us what to expect!
- Sat Mar 06, 2021 9:05 pm
- Forum: Student Social/Study Group
- Topic: Review Sessions
- Replies: 11
- Views: 1034
Re: Review Sessions
I highly recommend Rosa and Wesleys' review sections. They're really good at explaining the conceptual parts of the class
- Sat Feb 27, 2021 5:36 pm
- Forum: Balancing Redox Reactions
- Topic: Determining which molecule is the oxidizing agent
- Replies: 49
- Views: 1983
Re: Determining which molecule is the oxidizing agent
You are correct. This concept was a little bit confusing for me in the beginning. Just remember that the oxidizing agent is NOT getting oxidized, and the reducing agent is NOT getting reduced.
- Sat Feb 27, 2021 5:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: coulomb?
- Replies: 18
- Views: 1166
Re: coulomb?
I think its important to remember that charge is measured in coulombs, while potential is measured in volts.
- Sat Feb 27, 2021 5:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E value
- Replies: 1
- Views: 133
E value
Conceptually, what does it mean when an E value is negative? I'm having a hard time understanding the concept and how it relates to E∘.
- Sat Feb 27, 2021 5:13 pm
- Forum: Balancing Redox Reactions
- Topic: sapling week 8 #18
- Replies: 4
- Views: 355
Re: sapling week 8 #18
This problem was tricky because I didn't remember learning how the dot symbol affects balancing the equation. I believe the dot combines the two molecules into "one unit." Any coefficient you put in front of the first part will affect the second half. If you try to balance them individuall...
- Sat Feb 27, 2021 5:08 pm
- Forum: Student Social/Study Group
- Topic: Chem Community Points
- Replies: 35
- Views: 1687
Re: Chem Community Points
The max is 5 posts weekly for 50 points total. Some TAs don't calculate the points weekly, so just check with yours what they prefer.
- Sat Feb 20, 2021 4:42 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Adiabatic
- Replies: 26
- Views: 1208
Re: Adiabatic
It is also good to know the difference between adiabatic and isothermal. Isothermal processes have no change in temperature. In an adiabatic process, the heat transferred is zero, but the change in internal energy will depend on if there is work.
- Sat Feb 20, 2021 4:30 pm
- Forum: Balancing Redox Reactions
- Topic: Pt in Cell Diagram
- Replies: 14
- Views: 949
Re: Pt in Cell Diagram
Chem_Mod replied in another post "In Chem 14B we'll keep it simple. Whenever a conducting electrode is needed, use Pt."
- Sat Feb 20, 2021 3:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 9
- Views: 1241
Re: Anode and Cathode
The way I like to remember anodes and cathodes is by relating them to anions and cations. Anions are negatively charged, while cations are positively charged (because CATs are awesome). Electrons are released at the anode during oxidation (LEO the lion, loss of electron to the CAThode) and goes to t...
- Sat Feb 20, 2021 3:07 pm
- Forum: Balancing Redox Reactions
- Topic: Chemical Reactions and Electrical Energy
- Replies: 6
- Views: 347
Chemical Reactions and Electrical Energy
Can somebody explain how a chemical reaction produces electrical energy?
- Sat Feb 20, 2021 3:01 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm 2 De-stressing
- Replies: 92
- Views: 7532
Re: Post Midterm 2 De-stressing
I ate ice cream and a slice of cheesecake to reward myself :)
- Fri Feb 12, 2021 11:07 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: ΔH and q
- Replies: 9
- Views: 479
ΔH and q
Can somebody explain under what circumstances the enthalpy ΔH is the same as q?
- Fri Feb 12, 2021 11:02 am
- Forum: Student Social/Study Group
- Topic: Studying for Midterm 2
- Replies: 45
- Views: 1993
Re: Studying for Midterm 2
Since there are so many formulas for this midterm, I recommend writing them down and making sure you know when to use them for different problems. Also, like the others have said, the step-up and review sessions are very helpful.
- Fri Feb 12, 2021 10:54 am
- Forum: Calculating Work of Expansion
- Topic: R constant
- Replies: 4
- Views: 213
Re: R constant
The change will be insignificant. I wouldn't worry about using it because the 5 digit isn't on the constants sheet. On the midterm you would just pick the closest answer if there is a slight decimal difference.
- Fri Feb 12, 2021 10:50 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous as Written
- Replies: 10
- Views: 564
Re: Spontaneous as Written
That is correct. It is also good to understand that if ΔSunivis positive, then ΔGrxn is negative. Therefore, the reaction would be spontaneous.
- Fri Feb 12, 2021 10:46 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: sapling 5/6 #9
- Replies: 4
- Views: 1065
Re: sapling 5/6 #9
It sounds like you're on the right track for the first part of the problem. Here is my equation: 2Al(s)+3Cl2(g)⟶2AlCl3(s)ΔH∘=−1408.4 kJ The change in entropy of the system is given by ΔS∘sys=∑S∘(products)−∑S∘(reactants), so you plug in the standard entropy values for this equation, multiply by their...
- Fri Feb 05, 2021 10:17 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Entropy
- Replies: 27
- Views: 1184
Re: Entropy
Entropy is also a state function that shows the number of microstates possible for the system through degeneracy. I think it'll be one of the key concepts to know for the next midterm because it relates to the second law of thermodynamics & there are several formulas relating to it on the consta...
- Fri Feb 05, 2021 10:08 pm
- Forum: Student Social/Study Group
- Topic: Midterm Review
- Replies: 7
- Views: 913
Re: Midterm Review
Like Kaitlyn said, the textbook is definitely the most important resource. If you really don't have time to do all of the problems, focus on the more difficult ones. If you can do the difficult ones by yourself, the easier ones shouldn't be a problem.
- Fri Feb 05, 2021 10:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work and system
- Replies: 3
- Views: 170
Re: Work and system
Also remember that if work is done on the system or heat is transferred into the system, then it is positive.
- Fri Feb 05, 2021 10:00 pm
- Forum: General Science Questions
- Topic: Careless Mistakes
- Replies: 54
- Views: 3845
Re: Careless Mistakes
This happens to me all the time. Before I do any chemistry work I always wash my face with cold water to really wake myself up. It also helps to double check the calculator inputs before pressing enter.
- Fri Feb 05, 2021 9:56 pm
- Forum: Student Social/Study Group
- Topic: Study routine.
- Replies: 51
- Views: 2702
Re: Study routine.
If you feel like you aren't learning anything new at the workshops, don't go to too many. Focus on the difficult textbook problems and make sure you can do those.
- Thu Jan 28, 2021 12:35 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Biological examples (ATP hydrolysis and osmotic pressure)
- Replies: 5
- Views: 553
Re: Biological examples (ATP hydrolysis and osmotic pressure)
Although this probably won't be tested specifically, it may be useful to know ATP hydrolysis is an exothermic reaction, and thus moves towards the reactants when exposed to heat. I believe this was briefly mentioned in the textbook.
- Thu Jan 28, 2021 12:31 am
- Forum: General Science Questions
- Topic: Careless Mistakes
- Replies: 54
- Views: 3845
Re: Careless Mistakes
When doing calculations, I always make sure to double check the values before moving on. Especially when using the calculator, make sure to check again before pressing the enter button. I've entered the wrong digits and decimal places into my calculator way too many times.
- Thu Jan 28, 2021 12:28 am
- Forum: Student Social/Study Group
- Topic: Midterm Practice
- Replies: 6
- Views: 384
Re: Midterm Practice
Here is the link for the Chem14B Pizza Rolls review !
viewtopic.php?f=160&t=58508&p=224708&hilit=pizza+rolls&sid=297c8e7a86a4e1bca675da3c35540d64#p224708
viewtopic.php?f=160&t=58508&p=224708&hilit=pizza+rolls&sid=297c8e7a86a4e1bca675da3c35540d64#p224708
- Thu Jan 28, 2021 12:25 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: bond enthalpies method
- Replies: 4
- Views: 231
Re: bond enthalpies method
To find delta H using bond enthalpies, you need to know the structure of the molecules. The problem will provide a value for each type of bond (ex: C-C, C=C). Looking at the reaction, see which bonds from the reactants are broken, and which bonds are then formed to make the product. To solve, take t...
- Thu Jan 28, 2021 12:19 am
- Forum: Student Social/Study Group
- Topic: Midterm Advice
- Replies: 3
- Views: 239
Re: Midterm Advice
Dr. Lavelle also mentioned out of the three methods for finding Delta H, bond enthalpies are the least accurate.
- Sat Jan 23, 2021 2:17 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: steam causing burns
- Replies: 40
- Views: 1407
Re: steam causing burns
The way I like to thing of it is that phase changes require energy to occur. As a result, steam will have more energy than liquid water. The extra stored energy that steam has because it is a gas will be released when it touches your skin and changes to a liquid, so it will burn more than liquid wat...
- Sat Jan 23, 2021 2:11 pm
- Forum: Student Social/Study Group
- Topic: Anxiety
- Replies: 109
- Views: 8178
Re: Anxiety
I always make sure to exercise when I feel anxious. It might seem like a lot of effort, but I guarantee you'll feel better afterwards.
- Sat Jan 23, 2021 2:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Studying for Midterm #1
- Replies: 63
- Views: 2840
Re: Studying for Midterm #1
Make sure you understand the conceptual topics. He usually includes a mixture of concept and calculating problems on the midterms. Personally, I re-do all of the homework problems before the exam.
- Sat Jan 23, 2021 2:01 pm
- Forum: Phase Changes & Related Calculations
- Topic: Elements Not in standard state
- Replies: 5
- Views: 171
Re: Elements Not in standard state
The important part to understand is that you would add the enthalpy of the phase change. I don't believe you will need to solve this type of question though because none of the textbook problems he assigned have it.
- Fri Jan 22, 2021 9:32 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Textbook 4D.3
- Replies: 3
- Views: 179
Textbook 4D.3
The reaction of 1.40 g of carbon monoxide with excess water vapor to produce carbon dioxide and hydrogen gases in a bomb calorimeter causes the temperature of the calorimeter assembly to rise from 22.113 8C to 22.799 8C. The calorimeter assembly is known to have a total heat capacity (calorimeter co...
- Sat Jan 16, 2021 11:06 am
- Forum: Polyprotic Acids & Bases
- Topic: What Does Monoprotic Mean?
- Replies: 16
- Views: 1073
Re: What Does Monoprotic Mean?
Monoprotic acids can only give away one proton, while polyprotic acids can donate more than one. I believe it is the same rule for monoprotic bases, where a monoprotic base can only accept one proton.
- Sat Jan 16, 2021 11:02 am
- Forum: Ideal Gases
- Topic: n/V = concentration
- Replies: 19
- Views: 2411
Re: n/V = concentration
Remember that molarity and concentration are basically the same thing. The formula for molarity is M=mol (n)/volume (L). So for the formula PV=nRT, we can divide both sides by volume and get P=MRT.
- Sat Jan 16, 2021 10:50 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Conjugate Seesaw Application
- Replies: 2
- Views: 146
Re: Conjugate Seesaw Application
I think you're applying it correctly. That was my understanding when Dr. Lavelle explained it.
- Sat Jan 16, 2021 10:45 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Weak versus Strong Acid and Base
- Replies: 10
- Views: 580
Re: Weak versus Strong Acid and Base
There are some guidelines to figure out if an acid or base is weak, but in general you will need to memorize the strong acids. The strong acids are HCl, HBr, HI, H2SO4, HNO3, HClO4, and HClO3. You can assume any other acid is not a strong acid. Additionally, acids with Carbon are usually weak. Stron...
- Sat Jan 16, 2021 10:38 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When x is negligible in Equilibrium constant
- Replies: 34
- Views: 1587
Re: When x is negligible in Equilibrium constant
It is safest to assume this only when K is less than 10^-4. Remember this is not the same as assuming x is zero in all cases, because we still need to solve x for the problem.
- Fri Jan 08, 2021 12:11 pm
- Forum: Student Social/Study Group
- Topic: Resources Outside of Class
- Replies: 6
- Views: 331
Re: Resources Outside of Class
If you don't fully understand the topics or just want a review, step-up sessions are good. If you mostly understand the content and want more practice problems, go to the workshop sessions. The drop-in sessions are for when you have either homework or concept questions.
- Fri Jan 08, 2021 11:59 am
- Forum: Ideal Gases
- Topic: PV=nRT and concentration
- Replies: 27
- Views: 1632
Re: PV=nRT and concentration
Molarity and concentration are the same for the purpose of these problems. Just remember that molarity is mol/volume (L), and "n" refers to moles.
- Fri Jan 08, 2021 11:47 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry Community Quota
- Replies: 16
- Views: 1133
Re: Chemistry Community Quota
They shouldn't expect you to make up for last quarter. Just inform your TA so they are aware when they input the grades.
- Fri Jan 08, 2021 11:44 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q>K
- Replies: 10
- Views: 371
Re: Q>K
You are correct that a reaction at equilibrium will not naturally make more products and have a Q value greater than the equilibrium constant. Examples of when Q will be higher are due to external factors (reactants have been removed) or the reaction has not yet reached equilibrium .
- Fri Jan 08, 2021 11:39 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5.35
- Replies: 2
- Views: 113
Textbook Problem 5.35
Number 35 says: The following plot shows how the partial pressures of reactant and products vary with time for the decomposition of compound A into compounds B and C. All three compounds are gases. Use this plot to do the following: (a) Write a balanced chemical equation for the reaction. (b) Calcul...
- Wed Dec 09, 2020 7:09 pm
- Forum: Conjugate Acids & Bases
- Topic: Amphoteric
- Replies: 11
- Views: 589
Re: Amphoteric
One of the UAs mentioned that most of the metalloid oxides such as Al2O3 are amphoteric.
- Wed Dec 09, 2020 7:05 pm
- Forum: General Science Questions
- Topic: study methods/recs
- Replies: 37
- Views: 2429
Re: study methods/recs
Since he said there will be textbook problems on the final, you should focus on those first. The review sections this week will definitely be helpful as well.
- Wed Dec 09, 2020 2:58 pm
- Forum: Polyprotic Acids & Bases
- Topic: Proton vs H+
- Replies: 14
- Views: 800
Re: Proton vs H+
Yes! H+ and proton are interchangeable. If you take the LS7 series they also reference H+ as protons.
- Tue Dec 08, 2020 12:58 am
- Forum: Bronsted Acids & Bases
- Topic: Textbook Fundamentals J.9
- Replies: 1
- Views: 159
Textbook Fundamentals J.9
Identify the salt that is produced from the acid–base neutralization reaction between (b) ammonia and phosphoric acid, Write the complete ionic equation for each reaction. I understand how to identify the salt, but I'm not sure how to write the ionic equation. Can anybody go through their thought pr...
- Tue Dec 08, 2020 12:31 am
- Forum: Bronsted Acids & Bases
- Topic: Textbook Fundamentals J.7
- Replies: 2
- Views: 354
Textbook Fundamentals J.7
Select an acid and a base for a neutralization reaction that results in the formation of (a) potassium bromide; (b) zinc nitrite; (c) calcium cyanide, Ca(CN) 2 ; (d) potassium phosphate. Write the balanced equation for each reaction. I understand how to do part A, but I'm stuck on part B. I don't un...
- Wed Dec 02, 2020 5:08 pm
- Forum: Bronsted Acids & Bases
- Topic: Strong Acid vs. Weak Acid
- Replies: 7
- Views: 442
Re: Strong Acid vs. Weak Acid
In general, strong acids have weaker bonds. Acids with Carbon are also weak. However, we will likely need to memorize the list because these trends are not inclusive of all types of acids.
- Wed Dec 02, 2020 5:05 pm
- Forum: Hybridization
- Topic: Pi Bonds Cannot Rotate
- Replies: 29
- Views: 4180
Re: Pi Bonds Cannot Rotate
Here is an image that may help you visualize it!


- Wed Dec 02, 2020 4:53 pm
- Forum: Bronsted Acids & Bases
- Topic: Why is HF a weak acid?
- Replies: 5
- Views: 20699
Re: Why is HF a weak acid?
HF is a weak acid because the bond between Hydrogen and Fluorine is so short. It is more difficult for the bond to be broken, so it will not completely dissociate in water.
- Wed Dec 02, 2020 4:48 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted Acids vs Strong Acids
- Replies: 4
- Views: 233
Re: Bronsted Acids vs Strong Acids
I believe weak acids can be characterized as Bronsted acids as well. As long as it donates protons it will be a Bronsted acid.
- Wed Dec 02, 2020 4:02 pm
- Forum: Bronsted Acids & Bases
- Topic: Comparing Acid Strength
- Replies: 1
- Views: 156
Comparing Acid Strength
Professor Lavelle mentioned in lecture that HBR is stronger than HCl. I just wanted to confirm that the weaker the bond is the stronger the acid is. So HAt would be a much stronger acid than HCl?
- Fri Nov 27, 2020 11:29 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: What does delocalized π bond mean?
- Replies: 18
- Views: 726
Re: What does delocalized π bond mean?
All double bonds have one pi bond. Since resonance structures are a blend of the possible structures (delocalized electrons), the pi bonds in a resonance structure would also be delocalized.
- Fri Nov 27, 2020 11:26 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelates
- Replies: 2
- Views: 124
Re: Chelates
It is important to know that it would more tightly connected due to multiple bonding sites.
- Fri Nov 27, 2020 11:19 am
- Forum: Hybridization
- Topic: Sp3d or dsp3
- Replies: 22
- Views: 2224
Re: Sp3d or dsp3
They both are correct. You can use either one of them.
- Fri Nov 27, 2020 11:17 am
- Forum: Electronegativity
- Topic: Periodic Table Trend
- Replies: 40
- Views: 2559
Re: Periodic Table Trend
Since Fluorine has the highest electronegativity, I try to remember the trend as the closer you get to Fluorine (top & right) the more electronegative.
- Fri Nov 27, 2020 11:13 am
- Forum: Student Social/Study Group
- Topic: Final Exam Tips
- Replies: 24
- Views: 1061
Re: Final Exam Tips
Definitely try to go to the UA sessions if you can. They're super helpful.
- Wed Nov 18, 2020 2:41 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Nodes in the d orbital
- Replies: 4
- Views: 436
Re: Nodes in the d orbital
I believe that is correct. One of the LAs informed me that the number of nodes is related to the "l" orbital angular momentum quantum number. Since the d orbital is "l"=2, it will have two nodal planes.
- Wed Nov 18, 2020 2:32 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Power/Polarizability Trend?
- Replies: 4
- Views: 854
Re: Polarizing Power/Polarizability Trend?
In general, polarizability will increase down and to the left because those elements have a larger radius and more electrons; so, Francium would have a high polarizability. Polarizing power increases as the size gets smaller and the charge of the cation increases. For example, an Aluminum 3+ cation ...
- Wed Nov 18, 2020 2:26 pm
- Forum: Hybridization
- Topic: 2.a.13 part d sappling
- Replies: 2
- Views: 179
Re: 2.a.13 part d sappling
Copper is one of the exceptions to the standard electron configuration rules. The other exception is chromium. Based on the rules we learned, it would appear copper's electron configuration would be [Ar] 4s^2, 3d^9. However, the d subshell would be very reactive because it only needs one more electr...
- Wed Nov 18, 2020 2:10 pm
- Forum: Resonance Structures
- Topic: Resonance
- Replies: 11
- Views: 716
Re: Resonance
It is also good to know that resonance structures are indicative of stability because they represent delocalization of electrons. They spread energy over a larger
area rather than keeping it confined to a small area.
area rather than keeping it confined to a small area.
- Wed Nov 18, 2020 2:00 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Multiple Lone Pairs in a Lewis Structure
- Replies: 1
- Views: 123
Multiple Lone Pairs in a Lewis Structure
Why is it when you draw H2O's Lewis structure you draw both lone pairs on the same side pushing down the bonds? I thought you were supposed to put the lone pairs as far away as possible like in XeF4's structure.
- Tue Nov 10, 2020 5:37 pm
- Forum: Student Social/Study Group
- Topic: Test Anxiety
- Replies: 62
- Views: 3500
Re: Test Anxiety
A good method to stay on track for a class is to make sure you truly understand all the material from one lecture before next class. That way you won't fall behind and feel overwhelmed. Right before starting a test if I feel nervous, I like to close my eyes for a few seconds and take a deep breath. ...
- Tue Nov 10, 2020 5:26 pm
- Forum: Bond Lengths & Energies
- Topic: Induced Dipole-Induced Dipole Interaction
- Replies: 3
- Views: 143
Induced Dipole-Induced Dipole Interaction
Can anybody explain the logic behind induced dipole-induced dipole interactions?
- Tue Nov 10, 2020 5:23 pm
- Forum: Bond Lengths & Energies
- Topic: Hydrogen Bonding
- Replies: 6
- Views: 421
Hydrogen Bonding
How is Hydrogen bonding different than normal bonding? I think he mentioned that Hydrogen can form a bond with a lone pair, but I didn't understand why this is possible.
- Tue Nov 10, 2020 5:03 pm
- Forum: Bond Lengths & Energies
- Topic: Dissociation Energy Trend
- Replies: 2
- Views: 117
Dissociation Energy Trend
Can anyone explain the dissociation energy trend in the periodic table? I want to make sure I understand it correctly.
- Tue Nov 10, 2020 4:50 pm
- Forum: Dipole Moments
- Topic: Polar vs Nonpolar
- Replies: 3
- Views: 177
Polar vs Nonpolar
Can anybody explain what makes a molecule polar vs. nonpolar and how it relates to dipoles? I know he mentioned it in lecture, but I didn't really understand it.
- Wed Nov 04, 2020 5:18 pm
- Forum: Student Social/Study Group
- Topic: Constants and Equations Page
- Replies: 8
- Views: 384
Re: Constants and Equations Page
The Rydberg constant (R) should be in the formula. On the online version its in a different font and italicized. It appears very lightly when printed, so maybe that's why it didn't appear on your sheet.
- Wed Nov 04, 2020 5:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Step Up Sessions
- Replies: 71
- Views: 7054
Re: Step Up Sessions
Agreed. I'm so glad Professor Lavelle organized these study sessions. The LAs have have been extremely helpful, and they always make great practice worksheets.
- Wed Nov 04, 2020 2:11 pm
- Forum: Lewis Structures
- Topic: Coordinate Covalent Bonds & Lewis Acid-base Reactions
- Replies: 6
- Views: 397
Coordinate Covalent Bonds & Lewis Acid-base Reactions
What type of reactions are acid-base reactions? Do coordinate covalent bonds only occur in Lewis acid-base reactions or can they occur in any molecule?
- Wed Nov 04, 2020 2:03 pm
- Forum: Lewis Structures
- Topic: Exception to Octet Rule
- Replies: 2
- Views: 114
Exception to Octet Rule
In the Lewis structure for BF3 , why can't there be a double bond with one of the Fluorine atoms? Is formal charge always more important than having an octet?
- Wed Nov 04, 2020 1:31 pm
- Forum: Lewis Structures
- Topic: Expanded Octet
- Replies: 6
- Views: 249
Expanded Octet
Can anybody explain which elements can have an expanded octet? In lecture he mentioned something about the d-block, but I didn't really understand.
- Mon Oct 26, 2020 5:40 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Potassium & Calcium Electron Configurations
- Replies: 1
- Views: 117
Potassium & Calcium Electron Configurations
In lecture Professor Lavelle mentioned that the 4s orbitals of Potassium and Calcium were occupied sooner than expected, but I don't really understand what it means when writing their electron configuration. Can anybody clarify?
- Mon Oct 26, 2020 5:15 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing Electron Configurations
- Replies: 5
- Views: 194
Writing Electron Configurations
Why do we write electron configurations from lowest energy to highest energy? Wouldn't writing it from highest to lowest give you more information at first glance?
- Mon Oct 26, 2020 4:53 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Degenerate Orbitals
- Replies: 3
- Views: 407
Degenerate Orbitals
Are degenerate orbitals just orbitals with the same energy? Is hydrogen the only atom that will have degenerate orbitals because its a one electron system?
- Mon Oct 26, 2020 4:22 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Wave Function Quantum Numbers
- Replies: 11
- Views: 354
Wave Function Quantum Numbers
Can anybody help explain the relationship the quantum numbers n, l, and m have with each other?
- Mon Oct 26, 2020 4:08 pm
- Forum: Properties of Light
- Topic: Lyman VS Balmer series
- Replies: 5
- Views: 253
Re: Lyman VS Balmer series
Yes, you're correct. Also remember that the Lyman Series corresponds with UV light, while the Balmer Series corresponds with visible light. Sometimes questions online provide the wavelength of the incident light, so memorize the general wavelength range for UV light & visible light.
- Thu Oct 22, 2020 5:24 pm
- Forum: SI Units, Unit Conversions
- Topic: Kilograms SI unit?
- Replies: 4
- Views: 158
Re: Kilograms SI unit?
Yes, kg is the SI unit for the formulas. Be careful because a lot of problems give values, especially electron or proton mass, in grams.
- Thu Oct 22, 2020 5:19 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Calculator
- Replies: 2
- Views: 97
Re: Calculator
The mode of the calculator shouldn't matter for this test. Just remember to try and use parentheses so there aren't errors when doing arithmetic with X10^x values.
- Thu Oct 22, 2020 5:14 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: What is a nodal plane?
- Replies: 4
- Views: 310
Re: What is a nodal plane?
Just think of a nodal plane as a place electrons won't be. You are correct only p, d, and f orbitals have a nodal plane, while the s orbital does not. Nodes are points of zero amplitude along a standing wave, which means there are no electrons present.
- Thu Oct 22, 2020 4:55 pm
- Forum: Properties of Light
- Topic: Wavelengths in Light Spectrum
- Replies: 9
- Views: 496
Re: Wavelengths in Light Spectrum
In general, you should how the wavelengths of each group relate to each other. The important ones to take note of are visible light (red and blue), UV light, microwaves, x-rays, gamma, and infrared. I doubt he would ask for the specific wavelengths without providing extra information.
- Thu Oct 22, 2020 4:50 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Conceptual difference between momentum and velocity
- Replies: 4
- Views: 558
Re: Conceptual difference between momentum and velocity
I definitely agree with what everyone else has been saying. Velocity is speed in a given direction, while momentum is the motion of an object. The equation illustrates that momentum is directly proportional to an object's mass and directly proportional to the object's velocity. Just be aware that a ...
- Thu Oct 22, 2020 4:41 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Hydrogen Emission Spectrum
- Replies: 3
- Views: 222
Hydrogen Emission Spectrum
I was confused by a practice problem from one of the TA's worksheets. An electric current is passed through a tube that contains Hydrogen gas. Light is then passed through the prism and the emission spectrum of Hydrogen gas is recorded. A band was recorded, and it has a frequency of 6.1684E14 hz. Wh...
- Tue Oct 13, 2020 6:17 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Uncertainty Principle
- Replies: 2
- Views: 214
Heisenberg Uncertainty Principle
What happens if 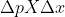 is less than Planck's/4pi? or is that not possible?
is less than Planck's/4pi? or is that not possible?
- Tue Oct 13, 2020 6:12 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Electromagnetic Spectrum
- Replies: 5
- Views: 170
Re: Electromagnetic Spectrum
You probably won't need to know the specific wavelengths, but definitely know the general size of each group compared to the rest. The common ones we will see are UV, X-rays, visible (know red and blue), microwaves, and infrared.
- Tue Oct 13, 2020 6:04 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Balmer & Lyman Series
- Replies: 2
- Views: 113
Balmer & Lyman Series
What do the Balmer & Lyman Series represent in atomic spectroscopy? Can anyone explain why they are important?
- Tue Oct 13, 2020 5:56 pm
- Forum: Photoelectric Effect
- Topic: Using Non-metals for the Photoelectric Effect? [ENDORSED]
- Replies: 2
- Views: 414
Using Non-metals for the Photoelectric Effect? [ENDORSED]
Does anyone know if you can only use metals for the photoelectric effect experiment? If so, why don't non-metals work for it?
- Tue Oct 13, 2020 5:49 pm
- Forum: Properties of Electrons
- Topic: Wavelike characteristics of objects
- Replies: 4
- Views: 215
Re: Wavelike characteristics of objects
Correct! It is way too small for us to observe. Wavelength is inversely proportional to mass, so the larger the object the smaller the wavelength.
- Wed Oct 07, 2020 5:48 pm
- Forum: Photoelectric Effect
- Topic: Textbook Example 1B.2
- Replies: 1
- Views: 79
Textbook Example 1B.2
The question asks what is (a) the energy of a single photon of blue light of frequency 6.4 X 10^14 Hz; (b) the energy per mole of photons of the same frequency. Once you calculate part a, how do you find the energy per mole of photons? The formula they used is E(per mole of photons) = N(subscript A)...
- Mon Oct 05, 2020 4:35 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Mol VS Mole
- Replies: 17
- Views: 794
Re: Mol VS Mole
Yes, they are both the same. Be careful not to confuse them as a shortened version of molecule, especially when doing conversions.

