Search found 84 matches
- Mon Mar 15, 2021 5:49 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Value of N
- Replies: 24
- Views: 1205
Re: Value of N
N, in this case, would be the number of electrons transferred in a redox reaction. It would make sense; in a way, they're the number of moles of electrons in a balanced equation. You'll be able to get that number if you balance a redox reaction by balancing the half-reactions!
- Mon Mar 15, 2021 11:33 am
- Forum: General Rate Laws
- Topic: Factors Affecting k
- Replies: 83
- Views: 5708
Re: Factors Affecting k
Yes, either by changing the temperature, or by changing the activation energy via a catalyst, as per the Arrhenius equation:
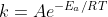
- Mon Mar 15, 2021 11:29 am
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Catalysts vs. Enzymes
- Replies: 8
- Views: 2220
Re: Catalysts vs. Enzymes
An enzyme is a biological catalyst, responsible for synthesizing and breaking down compounds in living organisms. So, in essence, all enzymes are catalysts, but not all catalysts are enzymes. Hope this helps!
- Fri Mar 12, 2021 3:27 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Collision Theory
- Replies: 5
- Views: 269
Re: Collision Theory
The collision theory is basically that in order for molecules to react, they have to collide with enough speed and in the right orientation (the way the molecules face each other) to cause a breaking of bonds.
- Thu Mar 11, 2021 2:06 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Concentration and the Rate constant
- Replies: 5
- Views: 280
Re: Concentration and the Rate constant
The concentration does affect the rate of the reaction, but NOT the rate constant. Things like temperature change and the presence of a catalyst do affect the rate constant.
- Thu Mar 11, 2021 2:03 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Lecture 26 Question [ENDORSED]
- Replies: 1
- Views: 121
Lecture 26 Question [ENDORSED]
Hello! When Dr. Lavelle showed us the pre-equilibrium approach, in the 2kK[NO]^2[O2] rate law, where did the 2 constant come from, and where did the 1/2 in 1/2(d[P]/dt) come from?
- Mon Mar 08, 2021 4:15 pm
- Forum: General Rate Laws
- Topic: Wk 9/10 Sapling #13
- Replies: 8
- Views: 456
Re: Wk 9/10 Sapling #13
When the fast equilibrium step is the first step, and the slow step the second, we need a substitution equation for the intermediate, the reactant of Step 2 that was the product of Step 1. A + B <=> C + D (fast eq) D + E ==> F + G (slow) We need a substitution for [D]. Luckily, since Step 1 is fast ...
- Mon Mar 08, 2021 4:08 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.1
- Replies: 1
- Views: 197
Re: 7D.1
The equation is actually ln(k2/k1) = -(Ea/R)(1/T2 - 1/T1). When we substitute that negative into the temperature change equation, the two temperatures get reversed, and it becomes (Ea/R)(1/T1 - 1/T2). Hope that helps!
- Wed Mar 03, 2021 4:47 pm
- Forum: First Order Reactions
- Topic: 0.693 ?
- Replies: 39
- Views: 8402
Re: 0.693 ?
0.693 = ln2
- Wed Mar 03, 2021 4:43 pm
- Forum: Zero Order Reactions
- Topic: When to use each order
- Replies: 19
- Views: 1157
Re: When to use each order
Algebraically, the order is the sum of the reactant concentrations' exponents. So if the rate = k[A][B], the reaction order would be 2 because 1+1=2
Graphically, the graph that is the most linear out of the three (A vs time, lnA vs time, 1/A vs time) is the reaction's order.
Graphically, the graph that is the most linear out of the three (A vs time, lnA vs time, 1/A vs time) is the reaction's order.
- Mon Mar 01, 2021 5:27 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Value of k throughout Experiments
- Replies: 5
- Views: 296
Re: Value of k throughout Experiments
k will be constant no matter the concentrations or the rates resulting from them; that's why you can use any experiment in a rate law problem to solve for k. It could, however, change depending on temperature.
- Mon Mar 01, 2021 5:25 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Kinetic vs thermodynamics
- Replies: 8
- Views: 565
Re: Kinetic vs thermodynamics
Kinetics is for how fast a reaction goes, and thermodynamics is for how favorable a reaction is, how much entropy changes, or how much heat is transferred. Hope this helps!
- Fri Feb 26, 2021 3:19 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Kinetic stability
- Replies: 14
- Views: 735
Re: Kinetic stability
Kinetic stability refers to the energy barrier for the reaction. Because the energy barrier for diamond to graphite is so large, the reaction, although spontaneous, is extremely slow that diamond virtually doesn't change. So, while diamond may be thermodynamically unfavorable, it's kinetically stable
- Thu Feb 25, 2021 11:14 am
- Forum: Administrative Questions and Class Announcements
- Topic: Which percentage corresponds with which grade?
- Replies: 8
- Views: 1392
Which percentage corresponds with which grade?
Hello! I'm wondering what percentage range corresponds with grades in this class. For instance, a B+ is from what percent to what percent? Is there a guide I can consult?
- Wed Feb 24, 2021 4:33 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Week 7/8 Sapling #9
- Replies: 3
- Views: 214
Re: Week 7/8 Sapling #9
Courtney Situ 2B wrote:Hello!
The problem specifies copper (I), and I believe you used the standard reduction potential for copper (II).
To save you the search, here it is:
Cu+(aq) + e– → Cu(s)
+0.52
Hope this helps!
Yes! Be sure to keep track of which ion variant's reduction potential you're using.
- Wed Feb 24, 2021 4:12 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Number 10
- Replies: 6
- Views: 352
Re: Sapling Number 10
I don't think there's a way around it; you'll have to know the reduction potentials. I'm sure they'll give you that kind of information should you be tested on this.
- Mon Feb 22, 2021 4:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge
- Replies: 30
- Views: 1444
Re: Salt Bridge
As the redox reaction progresses in the cell, there would start to be a more positive side and a more negative side, and that would persist if it weren't for the salt bridge. The salt bridge is meant to keep the charges balanced across the whole cell.
- Mon Feb 22, 2021 4:19 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing vs Reducing
- Replies: 55
- Views: 2674
Re: Oxidizing vs Reducing
I make sure to remember that it's swapped. The substance that is gaining electrons (and is being reduced) is the oxidizing agent, and the substance that is losing electrons (and is oxidized) is the reducing agent.
- Sat Feb 20, 2021 6:07 am
- Forum: Calculating Work of Expansion
- Topic: Work Formula
- Replies: 15
- Views: 837
Re: Work Formula
Delta n is just the change in moles of gas, since it's the ideal gas law. Hope this helps.
- Sat Feb 20, 2021 6:03 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 9
- Views: 1241
Re: Anode and Cathode
The anode is where oxidation happens, and electrons are released, which will flow to the cathode. The cathode is where reduction happens.
- Wed Feb 17, 2021 4:02 pm
- Forum: Balancing Redox Reactions
- Topic: Redox Charges
- Replies: 3
- Views: 256
Redox Charges
How do we know what charge (or oxidation number) an ion will have after a redox reaction? For example, how would we know that Mn 2+ is formed and not any other charge? Or would they tell us from the get go?
- Wed Feb 17, 2021 4:00 pm
- Forum: Balancing Redox Reactions
- Topic: Redox Mnemonic
- Replies: 11
- Views: 1071
Re: Redox Mnemonic
That helps plenty! I remember both LEOGER and OILRIG.
- Wed Feb 10, 2021 3:51 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Self-Test 4H.1A
- Replies: 2
- Views: 127
Re: Self-Test 4H.1A
Yes, pressure is inverse to volume, so for all other conditions being constant, if pressure decreases, volume increases, and so does entropy.
- Wed Feb 10, 2021 3:41 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Complex Moleclues [ENDORSED]
- Replies: 3
- Views: 149
Re: Complex Moleclues [ENDORSED]
I believe it has to do with the size of the substance/molecule as well as the number of atoms bonded that the molecule has. C4H10 is more complex and will have a higher entropy than C2H6. Intermolecular forces may also play a role, but I'm not sure. Hope this helps!
- Mon Feb 08, 2021 4:08 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Sapling Week 3/4 #18
- Replies: 3
- Views: 180
Re: Sapling Week 3/4 #18
Cp and Cv are the pressure-constant molar heat and the volume-constant molar heat, respectively.
I haven't used Cv for the delta U portion, though. I just got the change in volume from PV=nRT, then plugged that into w = -P(delta V) and converted that into joules. Afterwards, I did the q + w equation.
I haven't used Cv for the delta U portion, though. I just got the change in volume from PV=nRT, then plugged that into w = -P(delta V) and converted that into joules. Afterwards, I did the q + w equation.
- Mon Feb 08, 2021 3:53 pm
- Forum: Calculating Work of Expansion
- Topic: Week 4 Sapling #14 Help
- Replies: 5
- Views: 246
Re: Week 4 Sapling #14 Help
For the moles part, use PV = nRT to get n because the rest of the variables have been given to you.
Now, for Path A, use -nRT ln(V2/V1) where V1 and V2 are the two volumes mentioned, and for Path B, use -P(delta V). Hope this helps!
Now, for Path A, use -nRT ln(V2/V1) where V1 and V2 are the two volumes mentioned, and for Path B, use -P(delta V). Hope this helps!
- Sat Feb 06, 2021 3:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Sapling Week 3 and 4, Q5
- Replies: 5
- Views: 974
Sapling Week 3 and 4, Q5
I'm stuck on question 5 of the Sapling homework due tomorrow (W3 and W4) about 2M + 3Cl2 => 2MCl3. Help would be appreciated!
- Fri Feb 05, 2021 4:31 pm
- Forum: Calculating Work of Expansion
- Topic: Spontaneous
- Replies: 26
- Views: 1587
Re: Spontaneous
If an equation is spontaneous then it will happen all by itself, without the need for you to add some kind of energy to make it happen. For example, a gas has a disposition to expand to the full volume of the container, so a gas taking up full volume is a spontaneous process.
- Wed Feb 03, 2021 4:45 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Solving for change in internal energy
- Replies: 6
- Views: 182
Re: Solving for change in internal energy
Q is negative when heat is released, but positive when heat is absorbed.
W is negative when work is done by the system, but positive when work is done on the system.
W is negative when work is done by the system, but positive when work is done on the system.
- Wed Feb 03, 2021 4:38 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy definition
- Replies: 37
- Views: 2658
Re: Entropy definition
Entropy is the measure of disorder in a system, and you can really observe entropy in, for example, a gas. A gas won't stay in a small cluster; it will spread out and take up the entire volume of its container, thus indicating an increase in disorder (randomness)
- Mon Feb 01, 2021 3:41 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: What P when solving for work?
- Replies: 5
- Views: 125
Re: What P when solving for work?
The external pressure, I presume since work has to do with the pressure exerted on the system.
- Mon Feb 01, 2021 3:37 pm
- Forum: Calculating Work of Expansion
- Topic: Gas Constant
- Replies: 13
- Views: 865
Re: Gas Constant
It depends on the units you're using, for example, for pressure (It differs whether or not you're using atmospheres, bar, Pascal, etc.)
- Wed Jan 27, 2021 5:29 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling Learning Week 3 and 4 Homework Question 10
- Replies: 4
- Views: 265
Re: Sapling Learning Week 3 and 4 Homework Question 10
What the other comments said. I believe it's mc(delta T) of the liquid water = fusion heat of ice + mc(delta T) of the ice
- Wed Jan 27, 2021 5:26 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Pressure and Systems
- Replies: 2
- Views: 169
Pressure and Systems
Can somebody help me clarify how pressure works depending on the type of system (open, closed, isolated)?
- Mon Jan 25, 2021 4:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive Property versus Intensive Property
- Replies: 5
- Views: 302
Re: Extensive Property versus Intensive Property
Extensive properties are dependent on the amount of the substance, such as the mass or number of moles. Intensive properties are not dependent on the amount of substance; you divided it by the amount, so it's a measure of heat capacity per 1 gram or 1 mole. Specific heat capacity stays the same thro...
- Mon Jan 25, 2021 2:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and K(c)
- Replies: 2
- Views: 138
K and K(c)
The textbook talks about the conversion between K and Kc, but I'm having trouble understanding the difference between the two, how to convert from one to the other, and which one I should use. Any help would be appreciated!
- Fri Jan 22, 2021 4:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies of diatomics vs Bond Enthalpies of Everything else
- Replies: 2
- Views: 92
Re: Bond Enthalpies of diatomics vs Bond Enthalpies of Everything else
For diatomic elements, the diatomic molecular form is the most stable form; thus, they naturally exist that way, and the bond connecting them doesn't change. However, in other elements, the bond's strength and properties may change depending on what molecule it's in or what state it is; that's why t...
- Fri Jan 22, 2021 4:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Preferred way to calculate enthalpy
- Replies: 6
- Views: 407
Re: Preferred way to calculate enthalpy
I believe Hess's law is used when working with a multi-step reaction, and standard enthalpy of formation is used for calculating the enthalpy of a one-step reaction. I'm sure the latter could also be used for multi-step though.
- Wed Jan 20, 2021 3:42 pm
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic v. Exothermic
- Replies: 139
- Views: 15441
Re: Endothermic v. Exothermic
I believe so; endothermic reactions will always have a positive enthalpy and exothermic reactions will always have a negative enthalpy.
- Wed Jan 20, 2021 3:35 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase change and temperature
- Replies: 6
- Views: 221
Re: Phase change and temperature
I believe it's because of the enthalpy of vaporization in reverse that comes from the vapor condensing into a liquid. The heat is being released when a gas condenses into a liquid (phase change); that's why the temperature wouldn't change at that time. A larger difference in heat released (more nega...
- Fri Jan 15, 2021 4:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: KA KB predicting trends (outline)
- Replies: 6
- Views: 360
Re: KA KB predicting trends (outline)
The higher the Ka, the stronger the acid, and the higher the Kb, the stronger the base!
- Wed Jan 13, 2021 4:38 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: How to calculate the pKa
- Replies: 14
- Views: 945
Re: How to calculate the pKa
pKa is defined as -log(Ka), Ka being the acidity constant, and "log" being log base 10.
If you were given pKa you would probably have to find Ka, in that case, it would be 10^(-pKa). Hope this helps!
If you were given pKa you would probably have to find Ka, in that case, it would be 10^(-pKa). Hope this helps!
- Wed Jan 13, 2021 4:36 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: What is the Conjugate Seesaw
- Replies: 11
- Views: 729
Re: What is the Conjugate Seesaw
The stronger the acid, the weaker the conjugate base, and the stronger the base, the weaker the conjugate acid. This is because, for example in strong acids, the proton transfer equilibrium favors the formation of H3O+ ions, therefore, the splitting of the H+ ion from the strong acid. Thus, the conj...
- Mon Jan 11, 2021 4:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Homework Week 1-Problem#2
- Replies: 5
- Views: 317
Re: Sapling Homework Week 1-Problem#2
It's always best to convert to molar concentrations first. Divide 0.920 by 5 and 0.150 by 5 and you get Initial concentration of SO3 = 0.184 M Equilibrium concentration of O2 = 0.030 M When you do ICE for the reaction, 0.184 <---> 0 0 -2x +2x +x ---------------------------------- 0.184-2x 2x 0.030 y...
- Mon Jan 11, 2021 4:07 pm
- Forum: Ideal Gases
- Topic: Pressure and partial pressure
- Replies: 12
- Views: 518
Re: Pressure and partial pressure
If there are multiple gases in one container, partial pressure is the pressure of a single type of gas. Total pressure is the combined pressure of all gases in a container. So if there was a jar filled with nitrogen, oxygen, and carbon dioxide, the pressure of oxygen in the jar would be a partial pr...
- Fri Jan 08, 2021 4:30 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increasing the Volume
- Replies: 5
- Views: 184
Re: Increasing the Volume
Yes! I've tried it out with a calculator, and when the volume is increased, the reaction will begin to favor the side with more moles of gas.
- Wed Jan 06, 2021 4:29 pm
- Forum: Ideal Gases
- Topic: PV=nRT
- Replies: 74
- Views: 4842
Re: PV=nRT
P = pressure
V = volume
n = moles
R = gas constant (around 0.08206 I believe)
T = temperature in Kelvin
V = volume
n = moles
R = gas constant (around 0.08206 I believe)
T = temperature in Kelvin
- Wed Jan 06, 2021 3:53 pm
- Forum: Ideal Gases
- Topic: What makes an ideal gas an ideal gas?
- Replies: 9
- Views: 374
What makes an ideal gas an ideal gas?
Is it just about PV equaling nRT, or is there a property about those gases that makes them ideal? I must have forgotten from the last time I did chem.
- Mon Jan 04, 2021 4:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp (pressure used as concentration?)
- Replies: 3
- Views: 119
Re: Kp (pressure used as concentration?)
I believe we plug in the moles of that specific gas into the ideal gas law, along with the total volume of the container, to get the partial pressure.
- Mon Jan 04, 2021 4:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids Not Having Concentration
- Replies: 7
- Views: 465
Re: Solids Not Having Concentration
Solutes and solvents' molar concentrations are capable of changing; however,
for pure substances such as solids and pure liquids, their concentrations would not change. That's why we leave them out of the equation.
for pure substances such as solids and pure liquids, their concentrations would not change. That's why we leave them out of the equation.
- Fri Dec 11, 2020 2:15 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3707353
Re: Post All Chemistry Jokes Here
I don't like getting acid all over me. Nothing really happens; it just gets under my skin.
- Wed Dec 09, 2020 3:27 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Acidic, basic, or neutral?
- Replies: 10
- Views: 1687
Re: Acidic, basic, or neutral?
It's all in whether or not the ions can form a strong acid/base or a weak acid/base. If both the acid and base are strong, the ion is neutral. If one's a weak acid and the other a strong base, the salt is basic. If one's a strong acid and the other a weak base, the salt is acidic.
- Wed Dec 09, 2020 3:21 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Titration Diagram
- Replies: 5
- Views: 253
Re: Titration Diagram
These points just represented the pH at different volumes; they're supposed to demonstrate the change in pH before and after the stoichiometric point
- Mon Dec 07, 2020 3:29 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Which salts are acids or bases?
- Replies: 4
- Views: 244
Re: Which salts are acids or bases?
Some salts are neutral salts, and they generally come from the reaction between a strong acid and a strong base. In addition, the ions of a neutral salt in water aren't effective in changing the pH of a solution.
- Mon Dec 07, 2020 2:28 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: neutralization reactions
- Replies: 5
- Views: 301
Re: neutralization reactions
That's how I've been comprehending it! I usually think of neutralization reactions between strong acids and strong bases in this formula: Acid + Base -> Salt + Water. It makes sense that strong acids and strong bases would produce water because all the strong bases have OH and the strong acids have...
- Fri Dec 04, 2020 3:17 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Acid strength and bond length
- Replies: 11
- Views: 2153
Re: Acid strength and bond length
HBr would be the stronger acid. The longer the bond in a binary acid, the stronger it will be. When the bond is weaker, the H+ ion is easier to remove, meaning the acid would release more H+ ions into a solution by dissociating. Hence, the acid would be stronger.
- Wed Dec 02, 2020 3:47 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric vs Amphiprotic
- Replies: 13
- Views: 1535
Re: Amphoteric vs Amphiprotic
All amphiprotic substances are amphoteric, because they all act like either acids or bases depending on the situation. However, not all amphoteric substances are amphiprotic, because amphiprotic substances donate or accept hydrogen ions, and some amphoteric substances don't have to donate or accept ...
- Wed Dec 02, 2020 3:31 pm
- Forum: Bronsted Acids & Bases
- Topic: Molarity and Strong Acid Ionization
- Replies: 3
- Views: 559
Re: Molarity and Strong Acid Ionization
HCl, as a strong acid, will dissociate in water, and the H+ ion will be accepted by H2O to create H3O+. The acid-base interaction is as follows: HCl + H2O <--> H3O+ + Cl- All it takes from here is stoichiometry. The ratio of HCl to H3O+ is 1:1, same with HCl to Cl-. That's why there is 0.1 M H3O+ an...
- Mon Nov 30, 2020 2:56 pm
- Forum: Biological Examples
- Topic: Hemoglobin vs. Myoglobin
- Replies: 30
- Views: 1370
Re: Hemoglobin vs. Myoglobin
Myoglobin transports oxygen in muscle tissue, whereas hemoglobin transports oxygen in blood. In addition, hemoglobin transports more oxygen than myoglobin because hemoglobin has four myoglobin-like structures that can carry oxygen. Hope this helps!
- Mon Nov 30, 2020 2:54 pm
- Forum: Naming
- Topic: Naming Conventions for Polyatomic Ion Ligands
- Replies: 2
- Views: 175
Naming Conventions for Polyatomic Ion Ligands
Are there any naming rules for polyatomic ions that are ligands in a coordination compound? I'm talking about chlorite, chlorate, phosphate, etc. They wouldn't just be called "chloro", right? That's the ligand name of chloride ions. What are the naming conventions besides adding "o&qu...
- Fri Nov 27, 2020 2:35 pm
- Forum: Hybridization
- Topic: Unhybridized Orbital
- Replies: 1
- Views: 64
Unhybridized Orbital
I'm still not sure as to why, for example, when carbon bonds with 3 atoms and one of the bonds is a double bond, how the unhybridized orbital forms. Are there simply not enough hybridized orbitals for four electrons?
- Wed Nov 25, 2020 3:29 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Homework question Sapling
- Replies: 6
- Views: 364
Re: Homework question Sapling
If it were linear and the two lone pairs cancel its forces out, then the angle between the lone pair and the bond would be 90 degrees, and electron repulsion wouldn't allow that. When there are four regions of electron density, the electron geometry is tetrahedral and the bond angles are 109.5 degre...
- Wed Nov 25, 2020 2:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Nature of Transition Metals
- Replies: 1
- Views: 78
Nature of Transition Metals
What is it about transition metal cations specifically, that are responsible for them binding to ligands, and how can it bind with 6 ligands? Does it work the same way as an ionic compound?
- Mon Nov 23, 2020 2:02 pm
- Forum: Hybridization
- Topic: regions of electron density
- Replies: 7
- Views: 403
Re: regions of electron density
The number of regions of electron density has to do with how many lone pairs and bonding areas there are (single, double, and triple bonds are counted as one bonding region). CH 4 has 4 bonds, therefore, 4 regions of electron density. Likewise, NH 3 has 3 bonds and 1 lone pair on nitrogen, making up...
- Mon Nov 23, 2020 1:59 pm
- Forum: Hybridization
- Topic: Bond Order and Hybridization
- Replies: 1
- Views: 114
Bond Order and Hybridization
I'm having trouble understanding the connection between the presence of sigma VS pi bonds, and the hybridization of orbitals. Assuming, of course, there is one.
- Wed Nov 18, 2020 9:29 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Covalent Character and Polarizability
- Replies: 4
- Views: 712
Covalent Character and Polarizability
I'm a little confused as to how stronger polarizing power (cation) and higher polarizability (anion) could result in the bond having more covalent character. If those forces are greater, I'd have assumed it would have more ionic character.
- Wed Nov 18, 2020 9:25 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability of Anions and Cations
- Replies: 6
- Views: 1028
Re: Polarizability of Anions and Cations
Generally smaller, highly-charged cations, such as Al 3+ , will have a greater polarizing power because of a greater effective nuclear charge, whereas larger anions will be more polarizable (i.e. their electron cloud will be more distorted), because their nuclei have a weaker pull on their valence e...
- Mon Nov 16, 2020 10:46 am
- Forum: Trends in The Periodic Table
- Topic: Why don't other np4 elements behave like oxygen in terms of ionization?
- Replies: 2
- Views: 152
Why don't other np4 elements behave like oxygen in terms of ionization?
I understand why oxygen is the exception to the trend of ionization energy and why it's lower. However, I want to clarify why other Group 16 elements (Sulfur, Selenium, etc.) don't have a lower ionization energy like oxygen? Or hold on, do they?
- Sat Nov 14, 2020 6:15 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3707353
Re: Post All Chemistry Jokes Here
I like talking to particles, but sometimes they just wave, and it gets really annoying.
- Sat Nov 14, 2020 6:09 pm
- Forum: Hybridization
- Topic: Hybridization and Steric Number [ENDORSED]
- Replies: 1
- Views: 192
Hybridization and Steric Number [ENDORSED]
What does steric number have to do with hybridization? I know how to assign hybridizations based on steric number, but does a larger steric number mean more hybrid orbitals?
- Sat Nov 14, 2020 6:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent Shape
- Replies: 31
- Views: 3016
Re: Bent Shape
The bent molecule shapes are either AX2E, or AX2E2, meaning the central atom would have either one or two lone pairs.
- Wed Nov 11, 2020 7:41 pm
- Forum: Bond Lengths & Energies
- Topic: Hydrogen Bonding
- Replies: 6
- Views: 421
Re: Hydrogen Bonding
Hydrogen bonding happens between molecules; the slightly positive hydrogen is attracted to the slightly negative side of a polar molecule. Normal bonding happens within a molecule and binds the atoms in a molecule together
- Wed Nov 11, 2020 7:36 pm
- Forum: Dipole Moments
- Topic: Dipole dipole vs LDF
- Replies: 10
- Views: 3463
Re: Dipole dipole vs LDF
London Dispersion forces happen between every type of molecule, because it has to do with the very brief polarizability of an electron field. Dipole-dipole forces have to happen between two polar molecules, because the unequal covalent bond makes the opposite ends of each molecule attract, pulling t...
- Mon Nov 09, 2020 3:48 pm
- Forum: Bond Lengths & Energies
- Topic: Negative energies? [ENDORSED]
- Replies: 7
- Views: 752
Re: Negative energies? [ENDORSED]
When ions interact, it's because the resulting compound would be more stable than if they were individual ions. That's why when they interact and form an ionic bond, they release energy.
- Fri Nov 06, 2020 3:50 pm
- Forum: Octet Exceptions
- Topic: Phosphorus and d-Orbital
- Replies: 7
- Views: 364
Re: Phosphorus and d-Orbital
I'm not sure, but I think it has to do with the d-subshell being on the same energy level (same quantum number) as 3p. The expanded octet works by using a different type of orbital.
- Fri Nov 06, 2020 3:47 pm
- Forum: Lewis Structures
- Topic: Difference in ionic and covalent Lewis structures
- Replies: 9
- Views: 2807
Re: Difference in ionic and covalent Lewis structures
Covalent Lewis structures are drawn how a normal Lewis structure is drawn, but ionic Lewis structures are drawn with the cation between brackets and the charge indicated, and the anion drawn the same way next to the cation structure. (E.x. [Li]+[| F |]-, I don't quite know how to type out a lewis st...
- Fri Nov 06, 2020 2:51 pm
- Forum: Ionic & Covalent Bonds
- Topic: Atomic Radius
- Replies: 38
- Views: 3223
Re: Atomic Radius
As we go across a period, the number of protons increase one by one, which would result in a progressively higher electrostatic attraction
- Fri Nov 06, 2020 2:48 pm
- Forum: Ionic & Covalent Bonds
- Topic: Electronegativity Difference Between 1.5 and 2
- Replies: 4
- Views: 248
Electronegativity Difference Between 1.5 and 2
In Dr. Lavelle's latest lecture, he gives a rough guide that electronegativity differences higher than 2 indicate ionic bonds, whereas electronegativity differences lower than 1.5 indicate covalent bonds. How about electronegativity differences between 1.5 and 2? If such bonds exist, what is the nat...
- Mon Nov 02, 2020 3:31 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: When to use formal charge or octet rule?
- Replies: 12
- Views: 824
Re: When to use formal charge or octet rule?
Usually the octet rule has to be kept in mind when showing a Lewis structure and when calculating formal charge, but formal charge is used to see which Lewis structure is the most stable. Also, some elements (those of level n=3) can expand their octet to make their formal charge 0. Phosphorus can fo...
- Mon Nov 02, 2020 3:19 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: "Charge Separation"?
- Replies: 3
- Views: 171
"Charge Separation"?
In a part of Dr. Lavelle's lecture, he mentions charge separation between sulfur and oxygen atoms in sulfate. What does that mean exactly? I feel like it's kind of making sense, but it's also far from my grasp. Would anyone clarify what he meant and what this means for formal charges?
- Wed Oct 21, 2020 3:09 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminacy for Electrons in Bohr's Model of Atoms?
- Replies: 3
- Views: 249
Indeterminacy for Electrons in Bohr's Model of Atoms?
I'd just finished watching the section of Dr. Lavelle's most recent lecture, about how electrons cannot be confined to the space of the nucleus because the indeterminacy of velocity would be way too high, higher than the speed of light. What would the indeterminacy equation look like for Bohr's mode...
- Mon Oct 19, 2020 2:45 pm
- Forum: Properties of Light
- Topic: Connection of De Broglie with Photoelectric Effect?
- Replies: 1
- Views: 93
Connection of De Broglie with Photoelectric Effect?
I was watching Dr. Lavelle's latest lecture, and his discussion of whether or not an object's De Broglie wavelength can be analyzed as a wave depending on how short it is. If they're too short, they can't be analyzed as waves (and are instead analyzed by particles, from my understanding.) Does that ...
- Mon Oct 12, 2020 2:15 pm
- Forum: Properties of Electrons
- Topic: Quantum Principles and Neuron Principles?
- Replies: 1
- Views: 180
Quantum Principles and Neuron Principles?
I have just started watching the October 12th lecture about quantum mechanics, and the explanation of quantum mechanics involving discrete amounts instead of continuous ones (e.x. electrons cannot be in between two energy levels), reminded me of a principle that has to do with the activity of neuron...
- Fri Oct 09, 2020 4:25 pm
- Forum: Properties of Light
- Topic: Why do salts with different cations burn different colors?
- Replies: 2
- Views: 871
Why do salts with different cations burn different colors?
I would like to know more about how salts burning in differently colored flames is related to how the electrons of an element's atoms release energy when they traverse through energy levels to the ground state. Is it that most electrons emit the energy wavelength equivalent to red when they're stimu...

