Search found 89 matches
- Mon Mar 15, 2021 7:02 pm
- Forum: Balancing Redox Reactions
- Topic: oxidation numbers
- Replies: 9
- Views: 696
Re: oxidation numbers
Look at a molecule and if there is an element such a oxygen that is always a charge of -2, you can determine the oxidation number based on the charge of the overall molecule. For example, NO 2 - , the oxidation number of N would be +3 since the two oxygens will add to a charge of -4, and if the whol...
- Mon Mar 15, 2021 6:39 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Activation Energy and Catalysts
- Replies: 7
- Views: 3541
Re: Activation Energy and Catalysts
i understand why adding a catalyst will decrease the activation energy for the forward reaction, but im a bit confused as to why it's the same for the reverse. can someone explain this please? A catalyst will reduce the activation energy for the entire reaction in general, both forward and reverse....
- Mon Mar 15, 2021 6:32 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Forward vs. Reverse
- Replies: 22
- Views: 2174
Re: Forward vs. Reverse
You can think of it as if it were reading a book for example. You go left to right. So the same thing applies to a reaction. The forward reaction will be how it is presented to you and read left to right. The reverse would be the same reaction but from right to left. Additionally, when looking at a ...
- Sat Mar 13, 2021 11:12 pm
- Forum: General Rate Laws
- Topic: Determining Intermediate Concentrations
- Replies: 6
- Views: 480
Re: Determining Intermediate Concentrations
If it is not in the overall rate law, it has to be an intermediate.
- Sat Mar 13, 2021 11:10 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Effect of Temp. on K constant
- Replies: 8
- Views: 577
Re: Effect of Temp. on K constant
If you consider how K is represented by the ratio of products to reactants, then yes, as previously stated since increasing temperature in an exothermic reaction will lead to more products being formed, the value of k will decrease accordingly.
- Sat Mar 13, 2021 11:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Profile
- Replies: 5
- Views: 419
Re: Reaction Profile
Is the reaction "hump" the same as the energy barrier? Yes I believe so. I am not sure if I have heard the term reaction "hump" but it sounds pretty intuitive. The reaction barrier is that little (or big) hill in the reaction profile that the reaction has to get over when beginn...
- Sat Mar 13, 2021 10:58 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Relation between k and activation energy
- Replies: 10
- Views: 5632
Re: Relation between k and activation energy
A clear way to look at the relationship between k and the activation energy, Ea, is through the Arrhenius equation, 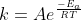 . This equation shows how a decrease in activation energy would ultimately lead to an increase in k and vice versa. Hope this helps!
. This equation shows how a decrease in activation energy would ultimately lead to an increase in k and vice versa. Hope this helps!
- Sat Mar 13, 2021 10:55 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Relation between k and activation energy
- Replies: 10
- Views: 5632
Re: Relation between k and activation energy
diangelosoriano wrote:If a catalyst were added, how would this also affect k or activation energy?
Adding a catalyst is going to decrease the activation energy, Ea, which will ultimately lead to an increase in the rate constant k.
- Sat Mar 13, 2021 10:48 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Catalysts vs. Enzymes
- Replies: 8
- Views: 2220
Re: Catalysts vs. Enzymes
Enzymes are proteins used in biological systems that act in the same way as catalysts. In that regard, they are the same thing as a catalyst; however, not all catalysts are enzymes necessarily.
- Sat Mar 13, 2021 10:46 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Temperature vs. activation energy
- Replies: 33
- Views: 2569
Re: Temperature vs. activation energy
Yes! If you look at the Arrhenius equation, k=Ae^{\frac{-E_{a}}{RT}} , you can clearly see how temperature affects the rate constant. If the temperature of the reaction is higher then the power to which e is multiplied will be a smaller negative number (aka more positive), and therefore the value of...
- Sat Mar 13, 2021 2:47 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Sapling 17
- Replies: 7
- Views: 648
Re: Sapling 17
If you draw out the reaction curve that shows the activation energy and whether it is exothermic or endothermic will be really helpful. Once you determine how the reaction works, you will be able to see how the activation energy, the delta H, and the reverse reaction are related. I believe since the...
- Sat Mar 13, 2021 2:41 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Arrhenius Eq
- Replies: 12
- Views: 758
Re: Arrhenius Eq
It isn't covered in lecture but I know there was a couple Sapling problems as well as textbooks examples that used it so it might be helpful for a problem or two on the final.
- Fri Mar 12, 2021 1:06 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Units for Activation Energy
- Replies: 28
- Views: 2007
Re: Units for Activation Energy
It is either kJ/mol or J/mol. Be careful about the units that are being dealt with and possibly the units of answer you are looking for. You may have a problem where you have to convert the given activation energy into either J/mol or kJ/mol
- Fri Mar 12, 2021 1:02 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow Step
- Replies: 14
- Views: 1412
Slow Step
Sorry if this is the wrong section to be posting this question but I am confused as to what the slow step is in a reaction and how to identify it? Why is it called the slow step?
- Fri Mar 12, 2021 1:00 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Exo or Endo based on Ea
- Replies: 4
- Views: 1669
Re: Exo or Endo based on Ea
If you know the E a for both the forward and reverse reaction, you could also determine if the reaction is exothermic or endothermic based on this. For example, if the E a for the forward reaction is greater than the E a for the reverse reaction, you would know that the forward reaction is endotherm...
- Fri Mar 12, 2021 12:56 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalyst Reverse Reaction
- Replies: 3
- Views: 269
Re: Catalyst Reverse Reaction
Ultimately, a catalyst simply lowers the activation energy of the reaction. Whether the forward reaction is exothermic or endothermic doesn't matter because the activation energy being lowered is applied to the entire reaction. So if you are looking at the reverse reaction, the activation energy is ...
- Fri Mar 12, 2021 12:51 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Are catalysts consumed?
- Replies: 37
- Views: 1886
Re: Are catalysts consumed?
Like others, this is based on my memory of high school biology class, but the catalyst is ultimately not consumed in a reaction. I believe that is why they are so important. They are able to change a slower reaction into a faster one without changing what products are formed. It is the same reaction...
- Fri Mar 12, 2021 12:47 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalyst vs temp increase
- Replies: 2
- Views: 213
Re: catalyst vs temp increase
Yes both increasing the temperature of a reaction and adding a catalyst will increase the rate constant. You can use the Arrhenius equation to determine that both factors would increase the rate constant. The Arrhenius equation, k=Ae^{\frac{-E_{a}}{RT}} , shows the relationship between the rate cons...
- Tue Mar 09, 2021 10:02 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Lecture 24 Example Intermediates
- Replies: 2
- Views: 296
Lecture 24 Example Intermediates
Hello I am super confused as to what Lavelle was talking about in his lecture 24 when talking about intermediates. I understand that 2NO 2 cannot form an NO and a CO 2 but I don't understand how in the steps he was showing, the NO and NO 2 just turn into CO and CO 2 . Can someone please clarify this...
- Fri Mar 05, 2021 7:10 pm
- Forum: Zero Order Reactions
- Topic: Sapling HW Week 9/10 #4
- Replies: 4
- Views: 426
Re: Sapling HW Week 9/10 #4
You need to look at how the elements that make up the rate constant. For example, when trying to find the units for the rate constant of the 3rd Order Reaction, look at the units of the rate(M/s) and the units of the concentration term(M 3 ).The unit of the rate constant is obtained by dividing the ...
- Fri Mar 05, 2021 12:04 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: First order reactions
- Replies: 3
- Views: 259
First order reactions
I am confused about what constitutes a reaction to be a first order reaction? Can there only be one reactant since the order is the value of the exponents added up? Additionally, how is it possible for there to be a zeroth reaction?
- Fri Mar 05, 2021 12:02 am
- Forum: General Rate Laws
- Topic: Gas not involved
- Replies: 3
- Views: 233
Gas not involved
I was a little confused when Lavelle brought up how gases leave the solution so therefore they are not included in the reverse reaction. Is this assuming that the system is open? Even if the system was closed would that even make a difference since it technically may still leave the solution even if...
- Wed Mar 03, 2021 9:06 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: ΔS total= 0
- Replies: 13
- Views: 1114
Re: ΔS total= 0
When the reaction is reversible, then the ΔSuniverse= 0
- Wed Mar 03, 2021 8:35 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: what does it mean when kinetics rather than thermodynamics is controlling a reaction
- Replies: 8
- Views: 520
Re: what does it mean when kinetics rather than thermodynamics is controlling a reaction
The diamond to graphite example is perfect for representing what "controlling a reaction" really means. Although the thermodynamic aspect of the reaction would suggest that the reaction of diamond to graphite should take place rather easily, there is a huge energy barrier in the way due to...
- Wed Mar 03, 2021 8:31 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Diamond vs. Graphite
- Replies: 23
- Views: 1122
Re: Diamond vs. Graphite
Yes, the reaction would take place eventually. Unfortunately since diamond is so kinetically stable, it would take a VERY long time, meaning it likely would not be observable, and it and would definitely not be very exciting.
- Wed Mar 03, 2021 8:23 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: kinetics vs. thermodynamics
- Replies: 23
- Views: 1293
Re: kinetics vs. thermodynamics
Saying a reaction is either kinetically or thermodynamically controlled means that the kinetic or thermodynamic factors of the reaction are ultimately determining if and how the reaction is going to take place. In this case, it can be helpful to look at what each term means. Thermodynamics looks at ...
- Wed Mar 03, 2021 8:15 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Reaction/Average Rate
- Replies: 13
- Views: 769
Re: Reaction/Average Rate
The average rate of the reaction is measured over a wide range of time. Although this average is useful, it becomes a more and more inaccurate representation of the reaction rate as the time range increases. This is where Lavelle introduced the instantaneous rate of change. The difference between th...
- Wed Mar 03, 2021 8:10 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Instantaneous Rate
- Replies: 41
- Views: 2269
Re: Instantaneous Rate
When considering that a reaction is beginning with a high concentration of reactants, the initial instantaneous rate will be very high as there are lots of reactants available; however, as the reaction continues as this high rate, the concentration of reactants will quickly decrease. With fewer reac...
- Wed Mar 03, 2021 8:06 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: -d[R]/dT versus d[P]/dt
- Replies: 13
- Views: 934
Re: -d[R]/dT versus d[P]/dt
These two equations will yield the same results if the concentration of each is the same and the stoichiometric coefficients are the same as well. The main difference will be the negative sign to indicate a difference in direction, but if you disregard the negative sign you could say the two rates a...
- Wed Mar 03, 2021 8:03 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 11456
Re: What was your favorite chem topic?
I like Kinetics so far! My favorite would probably have to be chemical equilibrium though.
- Wed Mar 03, 2021 7:52 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Kinetic control
- Replies: 14
- Views: 933
Re: Kinetic control
An example that Lavelle gave in lecture was how carbon in the form of a diamond is not as thermodynamically favored as carbon in the form of graphite; however, because of the large energy barrier between the two forms of carbon, the carbon will likely stay in diamond form as becoming graphite would ...
- Wed Mar 03, 2021 7:47 pm
- Forum: General Rate Laws
- Topic: unique average reaction rate change
- Replies: 4
- Views: 431
Re: unique average reaction rate change
As previously mentioned, the unique reaction rate would decrease by a factor of 2. In general, you can apply this same relationship (double coefficients ===> decrease by factor of 2) which any other change that could be made to the coefficients. For example, tripling the coefficients would decrease ...
- Thu Feb 25, 2021 9:35 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Types of Batteries
- Replies: 8
- Views: 586
Re: Types of Batteries
The difference is that rechargeable batteries utilize reversible chemical reactions that can be "fueled" by the power you can get from a power outlet for example. This allows for energy to be transferred back into the battery.
- Thu Feb 25, 2021 12:45 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: concentration and cell potential
- Replies: 3
- Views: 270
Re: concentration and cell potential
Introducing Le Chatlier's Principle was meant to demonstrate how increasing certain elements of a reaction will ultimately favor the increase of products or reactants, therefore changing the value of E cell . For example (same example given in lecture): 2Al (s) + 3Mn 2+ (aq) ===> 2Al...
- Thu Feb 25, 2021 12:30 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E cell vs Ecell naught
- Replies: 25
- Views: 1776
Re: E cell vs Ecell naught
The naught represents the variable (in this case E or cell potential) under standard conditions! These standard conditions are 1atm, 298 K and 1M. The Ecell represents the cell potential under the current conditions which are going to be different from the standard conditions in some way.
- Thu Feb 25, 2021 12:23 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation @ equilibrium
- Replies: 6
- Views: 374
Re: Nernst Equation @ equilibrium
Here is how Lavelle put it in lecture:
when a reaction is at equilibrium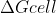 = 0
= 0
and since
then Ecell has to equal 0 in order for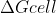 to equal 0 because n and F are both going to be positive constants.
to equal 0 because n and F are both going to be positive constants.
Hope this helps!
when a reaction is at equilibrium
and since
then Ecell has to equal 0 in order for
Hope this helps!
- Thu Feb 25, 2021 12:15 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Ecell vs E°cell
- Replies: 12
- Views: 1558
Re: Ecell vs E°cell
E° represents the cell potential at standard conditions. The naught (°) symbol attached to any other variables (H, G, S, etc.) means that it is that specific variable but at standard conditions (just as a rule of thumb for future reference :) ). As previously mentioned, these standard conditions are...
- Fri Feb 19, 2021 1:07 am
- Forum: Calculating Work of Expansion
- Topic: Value of Q
- Replies: 20
- Views: 1016
Value of Q
When looking at the equation w(max)= ) , I was confused as to what Q is? I understand it is the reaction quotient but how exactly do you calculate that?
, I was confused as to what Q is? I understand it is the reaction quotient but how exactly do you calculate that?
- Fri Feb 19, 2021 12:52 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy related to Enthalpy
- Replies: 20
- Views: 1005
Entropy related to Enthalpy
In outline 4, there is a bullet saying we should know how to "show how ∆S is related to ∆H for a change at constant temperature and pressure and explain the relationship". I am confused as to which equation this is referring to and wanted to make sure I understand the right connection I sh...
- Thu Feb 18, 2021 11:53 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Outline 4 Question Applying 1st and 2nd Laws
- Replies: 1
- Views: 120
Outline 4 Question Applying 1st and 2nd Laws
Could someone explain how to apply the 1st and 2nd laws of thermodynamics to calculate the change in energy and entropy of a
system? I am confused about what this is asking in specific, in terms of the equations and laws in place.
system? I am confused about what this is asking in specific, in terms of the equations and laws in place.
- Thu Feb 18, 2021 11:46 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Calculating degeneracy
- Replies: 18
- Views: 3523
Re: Calculating degeneracy
Just to clarify, the direction of a molecule also falls under the category of position? Meaning it could be in the same exact spot but facing a different direction and still count as a separate position?
- Thu Feb 18, 2021 11:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimetry question outline 3
- Replies: 2
- Views: 176
Re: Calorimetry question outline 3
Would a mass of some sort also be required for me to find the amount of heat released? I say this thinking that I would need to use the equation q=m*c*(delta)t
- Thu Feb 18, 2021 10:59 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimetry question outline 3
- Replies: 2
- Views: 176
Calorimetry question outline 3
I am confused about the wording of one of the bullets in outline 3. It says "Determine the heat output of a reaction, given the temperature change of a calorimeter". What does this mean?
- Sun Feb 14, 2021 8:18 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: confusion with notation
- Replies: 11
- Views: 566
confusion with notation
Hi this is a pretty simple question but I am becoming a little confused about this as I watch lectures. When Lavelle is writing certain equations, he's been writing d(something), for example dS= dq(rev)/T. Does d represent delta or is it something else?
- Sat Feb 13, 2021 6:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Explaining Enthalpies
- Replies: 7
- Views: 464
Re: Explaining Enthalpies
Hello I found this link that talks about some of the basics of enthalpy:
https://www.thoughtco.com/definition-of ... d%20as%20h.
hope this helps!
https://www.thoughtco.com/definition-of ... d%20as%20h.
hope this helps!
- Sat Feb 13, 2021 6:48 pm
- Forum: Phase Changes & Related Calculations
- Topic: Week 5/6 Sapling Question 7
- Replies: 5
- Views: 337
Re: Week 5/6 Sapling Question 7
Your problem is coming from the calculated difference in the masses. Switch the order of subtraction so that your value is positive and you should get the right answer.
- Sat Feb 13, 2021 6:45 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Microstates
- Replies: 16
- Views: 1116
Re: Microstates
I am confused about how to identify microstates. In Lavelle's lecture, he consistently used 2 for the value of microstates but I am assuming that is not always the case. In a given problem or example, what are you looking for when trying to identify the number of microstates?
- Sat Feb 13, 2021 5:20 pm
- Forum: Ideal Gases
- Topic: R ideal gas constant
- Replies: 31
- Views: 2188
Re: R ideal gas constant
Base it off of the units given/ the units you want for your answer. This will determine which R value you should use.
- Fri Jan 15, 2021 3:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 1 #9
- Replies: 7
- Views: 396
Sapling Week 1 #9
I am super confused on this problem because even after setting up my ice box, and calculating for x in multiple ways, I keep getting the same answer (0.0375). Subtracting this from 0.900 gets me 0.8625. I have tried changing the sig figs and everything but it is still telling me I am wrong. Am I doi...
- Fri Dec 11, 2020 8:32 pm
- Forum: Bond Lengths & Energies
- Topic: Boiling vs. Melting Point
- Replies: 15
- Views: 2876
Re: Boiling vs. Melting Point
Just adding on, and I am not entirely certain if this is what the original question is asking for, but in the context of bonds, the boiling and melting points are heavily affected by the strength of the bond. Specifically, the stronger the bond length is, the higher both the melting and boiling poin...
- Fri Dec 11, 2020 8:17 pm
- Forum: Coordinate Covalent Bonds
- Topic: Definition
- Replies: 4
- Views: 434
Definition
What exactly is a ligand in the context of chemistry? Sorry if this is a simple question I just have the biological definition of a ligand stuck in my head and it's confusing me. They're somewhat similar right?
- Fri Dec 11, 2020 8:13 pm
- Forum: Trends in The Periodic Table
- Topic: Finding Charge
- Replies: 4
- Views: 440
Re: Finding Charge
Yes, as previously stated when you are being asked for the charge of a transition metal, it should almost be within the context of the TM in a compound, otherwise it is hard to determine since their charges can vary. Use any element in the compound that you know the given charge of, as well as the o...
- Fri Dec 11, 2020 8:10 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: shielding and penetrating
- Replies: 5
- Views: 565
Re: shielding and penetrating
Also I think it is important to notice that other electrons contribute to the shielding effect with the negative repulsion. I found this on a website and I thought it was helpful: "In hydrogen-like atoms, which have just one electron, the net force on the electron is as large as the electric at...
- Fri Dec 11, 2020 8:00 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Location of nodes on a plane for D orbitals
- Replies: 2
- Views: 644
Re: Location of nodes on a plane for D orbitals
Hi I also found this snippet from this website: "There are four nodes total (5-1=4) and there are two angular nodes (d orbital has a quantum number ℓ=2) on the xz and zy planes. This means there must be two radial nodes. The number of radial and angular nodes can only be calculated if the princ...
- Fri Dec 11, 2020 7:53 pm
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: Buffer Definition
- Replies: 4
- Views: 1477
Buffer Definition
Could someone clarify for me what a buffer exactly? I am confused as to both what they are and what their intended purpose is? Also, how do you identify them?
Thanks
Thanks
- Fri Dec 11, 2020 1:09 am
- Forum: Resonance Structures
- Topic: Resonance Hyrbid
- Replies: 11
- Views: 650
Re: Resonance Hyrbid
Yes I believe stability is the main factor for why hybridization takes place. Without hybridization taking place, I believe the overall energy level would be higher and the likelihood of there being instability increases.
- Fri Dec 11, 2020 1:02 am
- Forum: Resonance Structures
- Topic: Lowest Energy Lewis Structure
- Replies: 8
- Views: 809
Re: Lowest Energy Lewis Structure
Adding on to this, since O is very electronegative does that mean it shouldn't be the central atom in lewis structures? Neha, the least electronegative atom is in the middle, and since nitrogen is less electronegative, it is the center atom. This is not to say though that oxygen can't be the centra...
- Fri Dec 11, 2020 12:57 am
- Forum: Octet Exceptions
- Topic: Octet Exceptions and Expanded Octet Calculation
- Replies: 3
- Views: 550
Octet Exceptions and Expanded Octet Calculation
Hi, I am a little confused as to where exactly the cutoff is for elements that are able to have more than 8 electrons in their valence shell. Also, I am confused about how to calculate what the central atoms expanded octet is when given a lewis structure or just a formula?
- Fri Dec 11, 2020 12:22 am
- Forum: Lewis Acids & Bases
- Topic: KA and pKA
- Replies: 19
- Views: 974
Re: KA and pKA
Kelly Singh wrote:KA measures the strength of an acid whereas pKA is the negative log of that value. It's just an easier and more convenient way to refer to the KA value as far as I know. =)
Does a higher Ka value represent a stronger acid or is it not that simple?
- Fri Dec 11, 2020 12:17 am
- Forum: Lewis Acids & Bases
- Topic: sapling #6
- Replies: 19
- Views: 969
Re: sapling #6
I thought the same thing but as previously mentioned, the COOH gives it away to be a weak acid. It dissociates partially into H+ and COO−. Here is a link that explains more about the carboxyl group: http://www.phschool.com/science/biology_place/biocoach/biokit/carboxyl.html#:~:text=Carboxyl%20groups...
- Fri Dec 11, 2020 12:11 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Delocalized
- Replies: 6
- Views: 1120
Re: Delocalized
I am still a little confused as to what causes delocalization in an atom or molecule? What could change an unstable molecule with uneven electron distribution into a stable one?
- Fri Dec 11, 2020 12:02 am
- Forum: Polyprotic Acids & Bases
- Topic: Calculating Ka
- Replies: 9
- Views: 1154
Re: Calculating Ka
So what is the exact equation for Ka? Also if only aqueous samples are used in calculating the Ka, then are any samples in different forms (solid, liquid, gas) just disregarded?
- Thu Dec 10, 2020 11:57 pm
- Forum: Polyprotic Acids & Bases
- Topic: How can you tell
- Replies: 18
- Views: 1036
Re: How can you tell
Sukhkiran_Kaur_3F wrote:If there is more than one H in front, it'll be polyprotic.
Is it specifically just in front or is it in general? I am a little confused as to whether or not all the H contribute to it being polyprotic?
- Thu Dec 10, 2020 11:22 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Sapling Week 10 #13
- Replies: 7
- Views: 439
Sapling Week 10 #13
For this question, I am confused as to how I am supposed to determine the pH of "the predominant species present at pH 2.70". How do I know what the predominant species is and how do I know what its pH is? How does the given pH and pKa help with that? Please help. Thank you.
- Thu Dec 10, 2020 11:18 pm
- Forum: Polyprotic Acids & Bases
- Topic: How can you tell if an acid/base is polyprotic?
- Replies: 6
- Views: 1031
Re: How can you tell if an acid/base is polyprotic?
I remember hearing about the term amphiprotic. What is the difference between amphiprotic and polyprotic? polyprotic is the ability of an acid to donate multiple protons (H+) while amphiprotic means a substance can act as an acid and a base. Yes, but not to confuse amphiprotic with amphoteric, whic...
- Thu Dec 10, 2020 10:51 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Categorizing Salts
- Replies: 3
- Views: 244
Categorizing Salts
What characteristics make a salt acidic or basic or neutral? Is it similar to normal acids and bases or is it different?
- Thu Dec 10, 2020 7:13 pm
- Forum: Bronsted Acids & Bases
- Topic: Sapling Week 10 #2
- Replies: 7
- Views: 581
Sapling Week 10 #2
I am confused as to how I can identify an amphoteric substance from a list of substances. I know that it means it the substance can act both as a Bronsted Acid or Base but how can I identify that just from looking at a list of substances?
- Wed Dec 09, 2020 3:09 pm
- Forum: Polyprotic Acids & Bases
- Topic: HCLO4 vs. H3PO4
- Replies: 4
- Views: 521
Re: HCLO4 vs. H3PO4
I am confused as to why something that is more electronegative would result in the formation of a weaker acid. Would the H+ not be more attracted to Cl- if it is so electronegative, thus making it harder to break away and therefore an overall weaker acid?
- Wed Dec 09, 2020 3:03 pm
- Forum: Conjugate Acids & Bases
- Topic: Conjugate Acids and Bases
- Replies: 4
- Views: 210
Re: Conjugate Acids and Bases
So basically to find the conjugate acid of a molecule, we add a hydrogen to it and give it a positive charge, and to find the conjugate base of a molecule, we take a hydrogen away from the molecule and make it a negative charge? Yes that is one way of looking at it for sure. I think focusing on how...
- Wed Dec 09, 2020 2:58 pm
- Forum: Air Pollution & Acid Rain
- Topic: Acid Rain applications
- Replies: 1
- Views: 264
Acid Rain applications
Does anyone know if we need to understand more than just how acid rain is formed? Or will we have to know more about the biological applications as well like how it negatively affects the environment etc.?
- Wed Dec 09, 2020 2:54 pm
- Forum: Conjugate Acids & Bases
- Topic: Amphoteric
- Replies: 11
- Views: 589
Re: Amphoteric
Amphoteric substances can act as either acids or bases, I think lavelle also said that the amphoteric substances often align with the diagonal band of metalloids, but that it doesn't match up perfectly. Can you clarify more on the "aligning with the diagonal band of metalloids"? Was he su...
- Wed Dec 09, 2020 2:50 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: pH formula?
- Replies: 69
- Views: 4103
Re: pH formula?
Also, when trying to find the concentration of the H+ or OH- from a pH or pOH, how do you do the reverse of -log[ ]?
- Wed Dec 09, 2020 2:46 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: pH formula?
- Replies: 69
- Views: 4103
Re: pH formula?
lwong Dis1L wrote:What is the difference between pH=-log[H3O+] and pH=-log[H+]?
They are the same thing. The H3O+ just represents the H+ bonded with an H2O but the concentration of either of them can be used to find the pH.
- Wed Dec 09, 2020 2:45 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: pH formula?
- Replies: 69
- Views: 4103
Re: pH formula?
Just to clarify, when using the concentration of OH to find the pH, you simply subtract -log[OH-] from 14 and that will equal the pH?
- Wed Dec 09, 2020 2:41 pm
- Forum: Bronsted Acids & Bases
- Topic: Telling whether something is an acid or a base
- Replies: 3
- Views: 259
Re: Telling whether something is an acid or a base
In regards to what the Chem Mod wrote about conjugate bases and acids, when an acid or base dissociates, is the portion that is not the H+ or the OH- the conjugate base and acid respectively? For example, ClO4- is the conjugate base but in the case of NaOH dissociating in H2O, the water is the conju...
- Wed Dec 09, 2020 2:35 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted Classification
- Replies: 2
- Views: 146
Bronsted Classification
What exactly makes an acid or a base a Bronsted acid and base? Are there unique characteristics to them compared to other types of acids and bases?
- Wed Dec 09, 2020 2:34 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Chem Final
- Replies: 7
- Views: 405
Re: Chem Final
Does anyone know what the format of the test is going to be like as well as how long the test is going to be?
- Tue Dec 01, 2020 10:26 am
- Forum: Naming
- Topic: Oxidation Number while Naming
- Replies: 9
- Views: 496
Re: Oxidation Number while Naming
Just to clarify, the roman numerals only apply to transition metal cations and anions? Also, the way you determine their charge is by looking at the charges of the other elements in the compound, as well as the total charge of the entire compound? Someone please correct me if I am wrong.
- Sat Nov 21, 2020 12:37 am
- Forum: Lewis Structures
- Topic: Identifying Lewis Acids and Bases
- Replies: 8
- Views: 505
Identifying Lewis Acids and Bases
I was wondering what are some helpful ways of remembering how to recognize which is a Lewis Acid and Lewis Base when presented with an equation for a reaction. For whatever reason I find it challenging to identify them when I look at an equation. Let me know!
- Sat Nov 21, 2020 12:33 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shape Names
- Replies: 50
- Views: 2574
Re: Shape Names
Like the person above mentions, there are a lot of really great graphics that display all the different shapes based on lone pairs and such. Here is a link to a website with a graphic that I find quite helpful: https://courses.lumenlearning.com/boundless-chemistry/chapter/molecular-geometry/ Hope th...
- Sat Nov 21, 2020 12:27 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: London Dispersion Forces
- Replies: 9
- Views: 1977
Re: London Dispersion Forces
Another question that I am confused about is how LDFs are affected by the molecule's surface area? If I am not mistaken, I believe that greater surface area means strong LDFs but why would that be the case?
- Sat Nov 21, 2020 12:20 am
- Forum: Dipole Moments
- Topic: Polarity
- Replies: 30
- Views: 1494
Re: Polarity
I understand that if there are multiple polar bonds present, and their dipole moments are not canceling out, then the molecule is polar. But what exactly makes a certain molecule more polar than another? Does it have to do solely with the strength of the polar bonds or are there other factors that c...
- Sat Nov 21, 2020 12:16 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Best Formal Charge Equations
- Replies: 24
- Views: 1145
Re: Best Formal Charge Equations
I agree with the previously mentioned equation using dots and lines. By counting each dot and line as 1, there is less confusion (at least for me) in terms of what value do I subtract from the valence electrons.
- Sat Nov 21, 2020 12:13 am
- Forum: Sigma & Pi Bonds
- Topic: Hybridization
- Replies: 7
- Views: 426
Re: Hybridization
Is there a specific benefit for hybridization? Why does it occur in the first place?
- Sat Nov 21, 2020 12:12 am
- Forum: Sigma & Pi Bonds
- Topic: Triple bond?
- Replies: 21
- Views: 908
Re: Triple bond?
In a triple bond, is there a way of knowing which specific bond is a sigma or a pi bond? Do we even need to know how to do this?
- Mon Oct 19, 2020 12:15 am
- Forum: Properties of Light
- Topic: Constructive and Destructive Interference
- Replies: 2
- Views: 113
Re: Constructive and Destructive Interference
I believe that overlapping waves will alter one another no matter where each wave is in its respective cycle. The obvious effects can be seen clearly at the peaks and troughs of the waves (when overlapped with other peaks and troughs), but I am sure that different effects can be noted at any point a...
- Mon Oct 19, 2020 12:08 am
- Forum: Properties of Electrons
- Topic: Electrons Excited or Ejected
- Replies: 19
- Views: 934
Re: Electrons Excited or Ejected
I believe "ejected" electrons are ones that have been removed from the atom due to the high frequency of the light being shone on it. "Excited" electrons are ones that are raised to higher levels of energy in the atom but do not leave. They eventually return to their original ene...
- Mon Oct 19, 2020 12:02 am
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 151903
Re: Reading the textbook
I ordered the textbooks required for Chem 14A from the UCLA bookstore but they are insanely back ordered I think because I still have not been contacted to get them. Does anyone know of another way to access the textbook? I know we are going to be needing it more and more as the quarter continues. T...
- Sat Oct 10, 2020 11:06 am
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 151903
Re: Reading the textbook
Has anyone else ordered a textbook from the campus bookstore and it still hasn't arrived? I am not really sure what to do about it because I already spent the money on it but we are going to be needing the textbooks more and more. Does anyone know of a place online where I can find it? Thanks.

