Search found 106 matches
- Sun Feb 28, 2021 8:24 am
- Forum: Ideal Gases
- Topic: K and Q
- Replies: 57
- Views: 3494
Re: K and Q
K=Q only when the reaction is at equilibrium. K is the constant at equilibrium, while Q can be the constant at any time. I think this website can help: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/E...
- Sun Feb 28, 2021 8:17 am
- Forum: Ideal Gases
- Topic: gas constant R
- Replies: 18
- Views: 1694
Re: gas constant R
Yes, it depends on the units.
There are 4 values that are used more often:
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
There are 4 values that are used more often:
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
- Sun Feb 28, 2021 8:14 am
- Forum: Ideal Gases
- Topic: The difference: ideal gas, ideal condition, ideal behavior
- Replies: 4
- Views: 819
Re: The difference: ideal gas, ideal condition, ideal behavior
I think there are several characteristics that are assumed for ideal gas: (1) the collisions between gas molecules are elastic and conservation of energy occurs among the molecules; (2) the total volume of the individual molecules is negligible comparing with the volume the gas occupies; (3) no inte...
- Sun Feb 28, 2021 8:05 am
- Forum: Balancing Redox Reactions
- Topic: Monoatomic Ions
- Replies: 2
- Views: 237
Re: Monoatomic Ions
Because it is ClO4- in KClO4 but it is Cl- in KCl.
- Sun Feb 28, 2021 8:01 am
- Forum: Balancing Redox Reactions
- Topic: oxidation states for final?
- Replies: 13
- Views: 742
Re: oxidation states for final?
For group 1 elements, the oxidation state is always +1. For group 2 elements, the oxidation state is always +2. Oxygen is usually -2, except in examples like H 2 O 2 and F 2 O. I think you can find more information on this website: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplem...
- Sun Feb 28, 2021 7:47 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Diamond vs. Graphite
- Replies: 23
- Views: 1122
Re: Diamond vs. Graphite
Yes, the activation energy is very high, so it would take very long for diamond to become graphite. There is more information about this on this website:
https://chemistry.stackexchange.com/questions/34193/are-diamonds-really-forever
https://chemistry.stackexchange.com/questions/34193/are-diamonds-really-forever
- Sun Feb 28, 2021 7:42 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Ranking Oxidizing Agents
- Replies: 5
- Views: 358
- Sun Feb 28, 2021 7:40 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E° vs. E and G° vs. G
- Replies: 25
- Views: 1081
Re: E° vs. E and G° vs. G
Yes. I think most of data are collected at STP, which is at 101.325kPa (1atm) and 0°C (273.15K), and when we try to calculate E or G at different temperatures or pressures, we can use the compare them with the E° and G° to get the answer.
- Thu Feb 25, 2021 12:03 pm
- Forum: Ideal Gases
- Topic: Pascal and Bar
- Replies: 4
- Views: 900
Re: Pascal and Bar
I think the values are:
1 atm = 101325 Pa = 101.325 kPa = 1.013 bar = 760 torr
1 atm = 101325 Pa = 101.325 kPa = 1.013 bar = 760 torr
- Thu Feb 25, 2021 11:59 am
- Forum: Ideal Gases
- Topic: STP
- Replies: 12
- Views: 1618
Re: STP
I think the values are:
Standard Temperature and Pressure (STP): 101.325kPa (1atm), 0°C (273.15K);
Standard Ambient Temperature and Pressure (STP): 101.325kPa (1atm), 25°C (298.15K).
Standard Temperature and Pressure (STP): 101.325kPa (1atm), 0°C (273.15K);
Standard Ambient Temperature and Pressure (STP): 101.325kPa (1atm), 25°C (298.15K).
- Thu Feb 25, 2021 11:56 am
- Forum: Ideal Gases
- Topic: 4.17 How to Identify When We Should Use the Ideal Gas Law?
- Replies: 4
- Views: 490
Re: 4.17 How to Identify When We Should Use the Ideal Gas Law?
I think if you are dealing with a chemistry problem, you can assume that the gas is an idea gas, and you can apply ideal gas law for most of the times. Nevertheless, the real characteristics of ideal gas should include: (1) the collisions between gas molecules are elastic and conservation of energy ...
- Thu Feb 25, 2021 10:49 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G v Delta G Naught
- Replies: 9
- Views: 512
Re: Delta G v Delta G Naught
You can still use the equation ΔG = ΔG。 + RTlnQ to derive.
- Thu Feb 25, 2021 10:36 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Combustion and Spontaneity [ENDORSED]
- Replies: 3
- Views: 324
Re: Combustion and Spontaneity [ENDORSED]
I think because combustion usually releases lots of heat, which means ΔH<0, and the reaction is very exothermic, ΔG would very likely be G<0.
- Thu Feb 25, 2021 10:31 am
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic v. Exothermic
- Replies: 139
- Views: 14796
Re: Endothermic v. Exothermic
ΔH<0, exothermic, releases heat;
ΔH>0, endothermic, absorbs heat;
ΔG<0, exergonic, spontaneous;
ΔG>0, endergonic, not spontaneous.
ΔH>0, endothermic, absorbs heat;
ΔG<0, exergonic, spontaneous;
ΔG>0, endergonic, not spontaneous.
- Thu Feb 25, 2021 10:17 am
- Forum: Ideal Gases
- Topic: Bars to atm [ENDORSED]
- Replies: 41
- Views: 1952
Re: Bars to atm [ENDORSED]
1 atm = 101325 Pa = 101.325 kPa = 1.013 bar = 760 torr
- Thu Feb 25, 2021 9:58 am
- Forum: Van't Hoff Equation
- Topic: How deltaG affects product/reactant formation
- Replies: 6
- Views: 741
Re: How deltaG affects product/reactant formation
It is not true that if there are more products than reactants, the formation of reactants would be favored. You can just think of an example of dissociation of a strong acid.
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
The products are favored even when there are more products than reactants.
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
The products are favored even when there are more products than reactants.
- Sun Feb 21, 2021 8:18 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: degeneracy and entropy
- Replies: 5
- Views: 369
Re: degeneracy and entropy
Kb = 1.38065 × 10−23J/K
- Sun Feb 21, 2021 8:14 am
- Forum: Ideal Gases
- Topic: Ideal Gas vs/ Real Gas
- Replies: 10
- Views: 1141
Re: Ideal Gas vs/ Real Gas
There are several characteristics that are assumed for ideal gas: (1) the collisions between gas molecules are elastic and conservation of energy occurs among the molecules; (2) the total volume of the individual molecules is negligible comparing with the volume the gas occupies; (3) no intermolecul...
- Sun Feb 21, 2021 8:10 am
- Forum: Biological Examples (*DNA Structural Transitions, etc.)
- Topic: STP [ENDORSED]
- Replies: 8
- Views: 2016
Re: STP [ENDORSED]
Standard Temperature and Pressure (STP): 101.325kPa (1atm), 0°C (273.15K);
Standard Ambient Temperature and Pressure (STP): 101.325kPa (1atm), 25°C (298.15K).
Standard Ambient Temperature and Pressure (STP): 101.325kPa (1atm), 25°C (298.15K).
- Sun Feb 21, 2021 7:50 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Equilibrium Systems
- Replies: 7
- Views: 410
Re: Equilibrium Systems
You can just treat other variables as constants to do the integral.
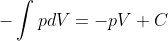
- Fri Feb 19, 2021 3:17 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Find Enthalpy: 2HCl (aq) + Zn (s) --> H2(g)+ ZnCl2 (aq)
- Replies: 3
- Views: 10731
Re: Find Enthalpy: 2HCl (aq) + Zn (s) --> H2(g)+ ZnCl2 (aq)
I think the equation is not correct. I think you should use
ΔHfo(Zn2+)+2ΔHfo(Cl-)-2ΔHfo(HCl)
which gives
-153.89 kJ/mol + 2 × (-167.16 kJ/mol) - 2 × (-167.16 kJ/mol)
and the result is
ΔHr = -153.89 kJ/mol
ΔHfo(Zn2+)+2ΔHfo(Cl-)-2ΔHfo(HCl)
which gives
-153.89 kJ/mol + 2 × (-167.16 kJ/mol) - 2 × (-167.16 kJ/mol)
and the result is
ΔHr = -153.89 kJ/mol
- Sun Feb 14, 2021 10:48 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Biological examples (ATP hydrolysis and osmotic pressure)
- Replies: 5
- Views: 551
Re: Biological examples (ATP hydrolysis and osmotic pressure)
ATP → ADP + Pi
ATP: adenosine triphosphate
ADP: adenosine diphosphate
Pi: phosphate ion
This reaction releases energy.
ATP: adenosine triphosphate
ADP: adenosine diphosphate
Pi: phosphate ion
This reaction releases energy.
- Sun Feb 14, 2021 10:37 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Temperature Question
- Replies: 8
- Views: 495
Re: Temperature Question
0K = -273.15°C
This, if we are considering a change in temperature (ΔT), 80 units in °C equal to 80 units in K.
This, if we are considering a change in temperature (ΔT), 80 units in °C equal to 80 units in K.
- Sun Feb 14, 2021 10:31 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: delta G
- Replies: 9
- Views: 570
Re: delta G
ΔG is free energy at a moment, while ΔGo is standard-state free energy.
ΔG = ΔGo + RTlnQ
ΔG = ΔGo + RTlnQ
- Sun Feb 14, 2021 10:28 am
- Forum: Ideal Gases
- Topic: Ideal Gas
- Replies: 10
- Views: 587
Re: Ideal Gas
There are several characteristics of ideal gases: (1) the collisions between gas molecules are elastic and conservation of energy occurs among the molecules; (2) the total volume of the individual molecules is negligible comparing with the volume the gas occupies; (3) no intermolecular forces exist ...
- Sun Feb 14, 2021 10:21 am
- Forum: Ideal Gases
- Topic: R ideal gas constant
- Replies: 31
- Views: 2176
Re: R ideal gas constant
It depends on the units in the problem. I think we should use the value of R whose units are the same as the units in the problem.
There are 4 values that are used more often:
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
There are 4 values that are used more often:
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
- Sun Feb 07, 2021 6:35 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong acids/bases
- Replies: 10
- Views: 598
Re: Strong acids/bases
Strong Acids: HCl, HBr, HI, (3 halogens)
HClO3, HClO4, (2 HClOn)
HNO3, H2SO4 (appears in problems very often)
Strong Bases: Group 1 hydroxides, Ca(OH)2, Sr(OH)2, Ba(OH)2, Group 1 and 2 oxides
HClO3, HClO4, (2 HClOn)
HNO3, H2SO4 (appears in problems very often)
Strong Bases: Group 1 hydroxides, Ca(OH)2, Sr(OH)2, Ba(OH)2, Group 1 and 2 oxides
- Sun Feb 07, 2021 6:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka2 << Ka1
- Replies: 12
- Views: 1159
Re: Ka2 << Ka1
Take H3PO4 as an example, Ka1=7.1 × 10-3, Ka2=6.3 × 10-8, Ka3=4.5 × 10-13. The difference between Ka1 and Ka2 is at the scale of 105. Thus, in these cases, the second deprotonation can often be ignored.
- Sun Feb 07, 2021 6:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pka vs ka
- Replies: 28
- Views: 1416
Re: pka vs ka
log10(ab) = log10(a) + log10(b) is correct
Also,
if pKw = pKa + pKb
10-pKw = 10-(pKa+pKb)
10-pKw = 10-(pKa) × 10-(pKb)
Kw = Ka × Kb
Also,
if pKw = pKa + pKb
10-pKw = 10-(pKa+pKb)
10-pKw = 10-(pKa) × 10-(pKb)
Kw = Ka × Kb
- Sun Feb 07, 2021 6:15 pm
- Forum: Ideal Gases
- Topic: Gas Constant Value
- Replies: 43
- Views: 1712
Re: Gas Constant Value
I think there are 4 values that are used more often:
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
- Sun Feb 07, 2021 6:13 pm
- Forum: Ideal Gases
- Topic: Units for K
- Replies: 29
- Views: 1276
Re: Units for K
I don’t think the units can cancel out all the time.
If A(aq) + B(aq) → 2C(aq) + D(aq)
Kc = [C]2 [D] / ([A] [B])
Because there is a square, one unit of [C] would not cancel out, and the unit of Kc should be the unit of [C].
If A(aq) + B(aq) → 2C(aq) + D(aq)
Kc = [C]2 [D] / ([A] [B])
Because there is a square, one unit of [C] would not cancel out, and the unit of Kc should be the unit of [C].
- Sun Jan 31, 2021 9:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reverse direction (sapling q.5)
- Replies: 7
- Views: 366
Re: Reverse direction (sapling q.5)
ΔHforward=-ΔHreverse
Thus, if you are given the ΔH for forward reaction and you wish to use the ΔH for reverse reaction, you should add a negative sign to the value.
Thus, if you are given the ΔH for forward reaction and you wish to use the ΔH for reverse reaction, you should add a negative sign to the value.
- Sun Jan 31, 2021 9:17 pm
- Forum: Ideal Gases
- Topic: Kp?
- Replies: 30
- Views: 1067
Re: Kp?
Yes, partial pressure only applies to gases. Just imagine adding a solid, a liquid, and a gas into a closed box. The solid and liquid would just occupy some volume of that box, but the gas would occupied the entire volume of that box. Therefore, only the gas would have partial pressure.
- Sun Jan 31, 2021 9:12 pm
- Forum: Calculating Work of Expansion
- Topic: Gas Constant
- Replies: 13
- Views: 863
Re: Gas Constant
It depends on the units you are using.
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
R = 0.0821 L·atm/(mol·K) = 8.3145 J/(mol·K) = 8.2057 m3·atm/(mol·K) = 62.3637 L·Torr/(mol·K)
- Sun Jan 31, 2021 9:06 pm
- Forum: Calculating Work of Expansion
- Topic: Extensive vs Intensive
- Replies: 4
- Views: 595
Re: Extensive vs Intensive
To give an example, heat capacity is an extensive property, while specific heat capacity is an intensive property. Heat capacity is the amount of heat given to a material with certain mass to make 1°C change in its temperature, while specific heat capacity is amount of heat needed to add to 1g of th...
- Sun Jan 31, 2021 8:57 pm
- Forum: Calculating Work of Expansion
- Topic: Which R to use?
- Replies: 4
- Views: 414
Re: Which R to use?
R = 0.0821 L·atm/(mol·K)
- Sun Jan 31, 2021 8:52 pm
- Forum: Calculating Work of Expansion
- Topic: Energy and the System/Surroundings
- Replies: 8
- Views: 291
Re: Energy and the System/Surroundings
Hello! I think posts above have already explained the first question very well, and I would just add a little bit information on the second question. The first law of thermodynamics states that energy cannot be destroyed or created (conservation of energy). Thus, it is very reasonable to think that ...
- Sun Jan 24, 2021 7:35 am
- Forum: Ideal Gases
- Topic: Ideal Gas Law as an Approximation
- Replies: 10
- Views: 996
Re: Ideal Gas Law as an Approximation
I think that means that we can easily see if we can apply PV=nRT in the question.
- Sun Jan 24, 2021 7:31 am
- Forum: Ideal Gases
- Topic: How Plausible is Ideal Gas?
- Replies: 2
- Views: 102
Re: How Plausible is Ideal Gas?
If the environment has higher temperature and lower pressure, the gas would act more ideal.
- Sun Jan 24, 2021 7:18 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Ionization Constant of water
- Replies: 4
- Views: 251
Re: Ionization Constant of water
Kw=1×10-14, so that pKw=14=pH+pOH at 25°C.
- Sun Jan 24, 2021 7:13 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Difference between Strong and Weak acids and bases
- Replies: 3
- Views: 210
Re: Difference between Strong and Weak acids and bases
Strong acids and bases would dissociate fully in water, but weak acids and bases would only dissociate partially.
- Sun Jan 24, 2021 7:10 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Direction of arrows
- Replies: 11
- Views: 547
Re: Direction of arrows
I think that means the equilibrium favors one direction very much. The reaction would favor where the arrow points very much.
- Sun Jan 17, 2021 9:13 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Section 6E
- Replies: 1
- Views: 92
Re: Textbook Section 6E
I think H2SO4 is an exception because its Ka2 = 1.0×10−2, which is close to other acids' Ka1, so it should not be ignored.
- Sun Jan 17, 2021 8:58 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Diprotic Acids
- Replies: 2
- Views: 118
Re: Diprotic Acids
I think in most of the times, yes. This is because the second Ka is often much smaller than the first Ka. Take H 3 PO 4 as an example, K a1 =7.1 × 10 -3 , K a2 =6.3 × 10 -8 , K a3 =4.5 × 10 -13 . The difference between K a1 and K a2 is at the scale of 10 5 . Thus, I think the second Ka can often be ...
- Sun Jan 17, 2021 8:53 am
- Forum: Polyprotic Acids & Bases
- Topic: Ignoring Second Deprotonation
- Replies: 2
- Views: 382
Re: Ignoring Second Deprotonation
Take H3PO4 as an example, Ka1=7.1 × 10-3, Ka2=6.3 × 10-8, Ka3=4.5 × 10-13. The difference between Ka1 and Ka2 is at the scale of 105. Thus, I think if it is not clarified specifically, the second deprotonation can often be ignored.
- Sun Jan 17, 2021 8:42 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Converting Qc to Qp [ENDORSED]
- Replies: 4
- Views: 1675
Re: Converting Qc to Qp [ENDORSED]
The way to calculate K c and Q c is very similar. Both are the relative ratio of products to reactants. The only difference is that K c is the relative ratio of products to reactants when the reaction is at equilibrium, while Q c is the relative ratio of products to reactants at a given instant (the...
- Sun Jan 17, 2021 8:23 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Quotient
- Replies: 12
- Views: 973
Re: Quotient
Basically, K is the relative ratio of products to reactants when the reaction is at equilibrium, while Q is the relative ratio of products to reactants at a given instant (the reaction might not be at equilibrium).
- Sun Jan 10, 2021 11:27 pm
- Forum: Lewis Structures
- Topic: Lewis Structure XeF_2
- Replies: 4
- Views: 2311
Re: Lewis Structure XeF_2
Nan_Guan_1L wrote:I think you can still draw the structure horizontally it doesn't really matter. as long as you represent the bond angle is roughly 180 degree I think it works either way you draw it.
I don't think you need to consider the bond angle when drawing a lewis structure.
- Sun Jan 10, 2021 8:20 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Using Gibbs free energy to calculate K
- Replies: 2
- Views: 448
Re: Using Gibbs free energy to calculate K
ΔGo=-RTlnK
- Sun Jan 10, 2021 8:16 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: atm
- Replies: 2
- Views: 604
Re: atm
I think just using same units in calculation would be fine. You can always convert units.
R=8.314 J mol-1 K-1=0.08205 L atm mol-1 K-1=62.363 mmHg L mol-1 K-1
R=8.314 J mol-1 K-1=0.08205 L atm mol-1 K-1=62.363 mmHg L mol-1 K-1
- Sun Jan 10, 2021 8:13 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Fall 2010, Question #6C (Equilibrium shifts right or left?)
- Replies: 4
- Views: 514
Re: Fall 2010, Question #6C (Equilibrium shifts right or left?)
According to the Le Chatelier's principle, the reaction would shift to counteract the change. Therefore, if more products are added, the reaction should shift towards the reactants because in this way, there will be less products and more reactants and the reaction counteracts the change of adding m...
- Sun Jan 10, 2021 8:03 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Converting between K and Kc
- Replies: 2
- Views: 587
Re: Converting between K and Kc
The formula should be easy to derive using PV=nRT:
P=nRT/V, because n/V is concentration,
P=cRT,
and because when calculating equilibrium constants, the stoichiometric coefficients should be the exponents, so take that into consideration,
KP=(RT)ΔnKc, Δn=nProducts-nReactants
P=nRT/V, because n/V is concentration,
P=cRT,
and because when calculating equilibrium constants, the stoichiometric coefficients should be the exponents, so take that into consideration,
KP=(RT)ΔnKc, Δn=nProducts-nReactants
- Sun Jan 10, 2021 7:58 am
- Forum: Ideal Gases
- Topic: Units for Pressure
- Replies: 41
- Views: 2584
Re: Units for Pressure
I don't think the unit of pressure should must be in atm. I think just using same units in calculation would be fine.
R=8.314 J mol-1 K-1=0.08205 L atm mol-1 K-1=62.363 mmHg L mol-1 K-1
R=8.314 J mol-1 K-1=0.08205 L atm mol-1 K-1=62.363 mmHg L mol-1 K-1
- Sun Jan 10, 2021 7:50 am
- Forum: Ideal Gases
- Topic: P over R in the Ideal Gas Law
- Replies: 4
- Views: 204
Re: P over R in the Ideal Gas Law
I think you should clarify if the K is K c or K P . I think if you are referring to K c , you should not assume that P/R is directly proportional to K c because when calculating K c , the concentrations are the bases while the stoichiometric coefficients are the exponents. The stoichiometric coeffic...
- Wed Dec 09, 2020 10:41 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: d-orbitals
- Replies: 2
- Views: 392
Re: d-orbitals
I think for d orbitals, there are dxy, dyz, dxz, dx2-y2, and dz2.
- Wed Dec 09, 2020 10:29 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration for Bi3+
- Replies: 2
- Views: 2215
Re: Electron Configuration for Bi3+
Because it should follow the order 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 5f 6d...
- Wed Dec 09, 2020 10:24 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration of Scandium
- Replies: 4
- Views: 821
Re: Electron Configuration of Scandium
It should follow the order 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 5f 6d... However, I think [Ar] 3d14s2 and [Ar] 4s23d1 are the same, and it does matter whether 4s or 3d is written first. They both represent the electron configuration of Sc.
- Wed Dec 09, 2020 10:17 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Valence-Shell Configurations
- Replies: 2
- Views: 841
Re: Valence-Shell Configurations
Valence shell configuration refers to the electrons in the outermost shell. The alkali earth metals are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra), and the respective electron configurations are [He] 2s 2 , [Ne] 3s 2 , [Ar] 4s 2 , [Kr] 5s 2 , [Xe] 6s 2...
- Wed Dec 09, 2020 10:02 pm
- Forum: Bronsted Acids & Bases
- Topic: Fundamentals J.7 b)
- Replies: 1
- Views: 137
Re: Fundamentals J.7 b)
The electron configuration of Zn is [Ar] 3d10 4s2. Therefore, we can infer that Zn would likely to lose the 2 electrons in 4s2 and form Zn2+. Therefore, as 2+2×(-1)=0, we should write Zn(OH)2 because it's Zn2+ and 2OH-.
Re: Cl & Br3
I think Br3- is tribromide, while Br- is bromide.
- Sun Dec 06, 2020 8:23 pm
- Forum: Lewis Structures
- Topic: Lewis Structure of XeO3?
- Replies: 3
- Views: 10337
Re: Lewis Structure of XeO3?
I think Kevin Wright’s answer is correct. Just to add on it, Xe forms 3 double bonds with 3 O, respectively, and has a lone pair electrons. Also, each O has 2 pairs of lone pair electrons.
- Sun Dec 06, 2020 8:06 pm
- Forum: Lewis Structures
- Topic: Lewis Structure for CH2
- Replies: 4
- Views: 6097
Re: Lewis Structure for CH2
I think its H — C: — H, and it’s called methylene.
- Sun Dec 06, 2020 7:59 pm
- Forum: Lewis Structures
- Topic: Lewis Structure for CO
- Replies: 3
- Views: 949
Re: Lewis Structure for CO
C has 4 valences electrons, O has 6 valence electrons. If there is a double bond in CO, C still needs 2 pairs of lone pair electrons to satisfy octet rule, and O also needs 2 pairs of lone pair electrons to satisfy the octet rule. Therefore, in total there should be 2×2+2×2+2×2=12 electrons. However...
- Sun Dec 06, 2020 7:51 pm
- Forum: Lewis Structures
- Topic: Valence Electrons
- Replies: 12
- Views: 1508
Re: Valence Electrons
Valence electrons are the electrons in the outer shell. If we use element carbon as an example. Carbon has the electron configuration [He]2s22p2. We can see that its outer shell is n=2, which is 2s and 2p. Thus, its valence electrons are 2+2=4.
- Sun Nov 29, 2020 5:15 am
- Forum: Lewis Structures
- Topic: Lewis Structure XeF_2
- Replies: 4
- Views: 2311
Re: Lewis Structure XeF_2
I do not think there is a reason. When drawing the Lewis structure, the same bond can be drawn in various ways (the bond angle does not matter). I think this is because Lewis structure is 2-D while the molecule is 3-D, therefore, there has to be some kind of distortion when trying to reflect the rea...
- Sun Nov 29, 2020 5:09 am
- Forum: Lewis Structures
- Topic: Molecular Shapes
- Replies: 5
- Views: 800
Re: Molecular Shapes
To add on NeelPatelLec4's answer, for seesaw, the bond angle between axial and axial is 180°, between equatorial and equatorial is 120°, and between axial and equatorial is 90°. For trigonal pyramidal, the bond angle should be less than 109.5° due to the repulsion from the lone pair electrons.
- Sun Nov 29, 2020 5:04 am
- Forum: Lewis Structures
- Topic: Bond Angles
- Replies: 2
- Views: 686
Re: Bond Angles
For seesaw structure, the bond angle between axial and axial is 180°, between equatorial and equatorial is 120°, and between axial and equatorial is 90°. For square planar structure, the bond angles should be 90°. These are the bond angles in ideal situations.
- Sun Nov 29, 2020 4:55 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 5
- Views: 304
Re: Bond Angles
Generally, lone pair electrons would make the bond angle to be smaller, because the lone pair electrons would have a repulsion force. For example, in a water molecule, there are 2 pairs of lone pair electrons, which makes the bond angle H-O-H to become 104.5°.
- Sun Nov 29, 2020 4:51 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent Shape molecules
- Replies: 7
- Views: 1094
Re: Bent Shape molecules
I think molecules that are AX2E1 or AX2E2 have a bent shape.
- Sun Nov 22, 2020 9:19 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Theory
- Replies: 5
- Views: 193
Re: VSEPR Theory
Yes, that means valence shell electron pair repulsion theory. Thus VSEPR theory.
- Sun Nov 22, 2020 8:42 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Model Memorization
- Replies: 3
- Views: 192
Re: VSEPR Model Memorization
Yes, the amount is determined, like for tetrahedral, there are always 4 atoms bonding to central atom and no lone pair electrons at central atom. However, I think it requires much work to memorize these. You can just draw the Lewis structure and determine the shape.
- Sun Nov 22, 2020 8:31 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Number of Molecular Shapes
- Replies: 6
- Views: 245
Re: Number of Molecular Shapes
I think we also have square pyramidal, pentagonal bipyramidal, pentagonal pyramidal, and others.
- Sun Nov 22, 2020 8:21 am
- Forum: Lewis Structures
- Topic: Lewis Structure for Glycine
- Replies: 3
- Views: 642
Re: Lewis Structure for Glycine
Glycine is an amino acid. Amino acid always has an amine group, a carboxyl group, and a side chain (R group). Also, There is an α carbon at the center that is connected to the amine group, the carboxyl group, and the side chain. I think if we know that glycine follows this structure, it would be pre...
- Sun Nov 22, 2020 8:12 am
- Forum: Lewis Acids & Bases
- Topic: How do I calculate expanded valence electrons?
- Replies: 3
- Views: 274
Re: How do I calculate expanded valence electrons?
The Cl atom in the middle forms 2 single bonds, and it has 3 lone pair electrons. Thus, the answer should be 10 valence electrons.
- Sun Nov 15, 2020 9:30 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing Electron Configurations
- Replies: 9
- Views: 888
Re: Writing Electron Configurations
I always use the order 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s...
- Sun Nov 15, 2020 9:16 am
- Forum: Lewis Structures
- Topic: Dispersion forces in CH₃ CHO
- Replies: 2
- Views: 150
Re: Dispersion forces in CH₃ CHO
Just as what is said in the Dr. lavelle's lecture, London Dispersion Force always exists because electrons always exist, and electrons always fluctuate.
- Sun Nov 15, 2020 8:57 am
- Forum: Coordinate Covalent Bonds
- Topic: OH
- Replies: 5
- Views: 461
Re: OH
OH- is not radical, •OH is radical.
- Sun Nov 15, 2020 12:58 am
- Forum: Ionic & Covalent Bonds
- Topic: A2.23 Textbook Problem
- Replies: 3
- Views: 229
Re: A2.23 Textbook Problem
We can consider how the element would lose or gain electrons in its outer shell. For example, Mg is [Ne] 3s2, so it has the tendency to lose 2 electrons, which makes Mg ion Mg2+, and Al is [Ne] 3s23p1, so it has the tendency to lose 3 electrons, which makes Al ion Al3+.
- Sun Nov 15, 2020 12:46 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Outline 2 Orbital Question
- Replies: 4
- Views: 234
Re: Outline 2 Orbital Question
We can consider the angular momentum quantum number. Electrons in s-, p-, d-, and f- orbitals have the angular momentum quantum number l = 0, 1, 2, and 3.
- Sun Nov 08, 2020 8:16 am
- Forum: Trends in The Periodic Table
- Topic: Understanding trends in the Periodic Table
- Replies: 2
- Views: 834
Re: Understanding trends in the Periodic Table
Yes, there is an easy method. You can simply distinguish the s-,p-, d-, and f- blocks by thinking about the graph. You can try to think like you want to cut the periodic table into rectangles. First you move He to the left to make a thin rectangle, making a 2×7 rectangle that represents s-block. The...
- Sun Nov 08, 2020 8:01 am
- Forum: Ionic & Covalent Bonds
- Topic: Salts and molecules
- Replies: 4
- Views: 198
Re: Salts and molecules
I would say yes, but one thing to be noticed is that, for example, NaCl(s) is a salt, but when it is dissolved in water, I would not call it a salt, because in water there are Na+ and Cl-, I would just call them sodium ion and chloride ion.
- Sun Nov 08, 2020 7:49 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 2A.1 Question
- Replies: 1
- Views: 122
Re: 2A.1 Question
This is because when we count valence electrons, we only count the electrons that are in the outer shell. The electrons whose principle quantum number is the greatest are in the outer shell. What does that mean? The electron configuration for Sb is [Kr]4d 10 5s 2 5p 3 , 5>4, that means the electrons...
- Sun Nov 08, 2020 7:40 am
- Forum: Lewis Structures
- Topic: 2B.11 part C
- Replies: 1
- Views: 89
Re: 2B.11 part C
It follows the same principle, but there are some tips. Carbon is always on the backbone, and for -COOH, it is always like C forms a single bond with an O, and for this O, it forms another single bond with H and has 2 pairs of electrons surrounding it, and for another O, it forms a double bond with ...
- Sun Nov 08, 2020 7:24 am
- Forum: Lewis Structures
- Topic: 2.B 5 part b
- Replies: 1
- Views: 84
Re: 2.B 5 part b
Br has 7 valence electrons while O has 6, and because it is BrO - , there should be 7+6+1=14 electrons in total. If there is a double bond, there should be 10 electrons that do not form bonds surrounding Br and O. When there is a double bond, Br allows 2 pairs of electrons around it, and O also allo...
- Sun Nov 01, 2020 5:09 am
- Forum: Significant Figures
- Topic: Sig Figs for electron shells
- Replies: 4
- Views: 320
Re: Sig Figs for electron shells
I think you can just add "Because electron shells are integers, the number is ...".
- Sun Nov 01, 2020 4:51 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Thorium and Silver
- Replies: 4
- Views: 245
Re: Thorium and Silver
Examples should include
Cu [Ar]3d104s1
and the elements around 4d and 5s
Y [Kr] 4d15s2
Zr [Kr] 4d25s2
Nb [Kr] 4d45s1
Mo [Kr] 4d55s1
Tc [Kr] 4d55s2
Ru [Kr] 4d75s1
Rh [Kr] 4d85s1
Pd [Kr] 4d10
Ag [Kr] 4d105s1
Cd [Kr] 4d105s2
Cu [Ar]3d104s1
and the elements around 4d and 5s
Y [Kr] 4d15s2
Zr [Kr] 4d25s2
Nb [Kr] 4d45s1
Mo [Kr] 4d55s1
Tc [Kr] 4d55s2
Ru [Kr] 4d75s1
Rh [Kr] 4d85s1
Pd [Kr] 4d10
Ag [Kr] 4d105s1
Cd [Kr] 4d105s2
- Sun Nov 01, 2020 4:33 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Shorthand Electron Configurations
- Replies: 6
- Views: 1709
Re: Shorthand Electron Configurations
It is always helpful to memorize the elements right after the noble gas elements.
Li [He]2s1
Na [Ne]3s1
K [Ar]4s1
Rb [Kr]5s1
Cs [Xe]6s1
Fr [Rn]7s1
Li [He]2s1
Na [Ne]3s1
K [Ar]4s1
Rb [Kr]5s1
Cs [Xe]6s1
Fr [Rn]7s1
- Sun Nov 01, 2020 4:17 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing out electron configurations
- Replies: 5
- Views: 236
Re: Writing out electron configurations
Yes, I agree with Sydney_Lam3G. For example, we can just write 1s22s22p6 for Ne.
- Sun Nov 01, 2020 4:10 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground State Electron Configuration
- Replies: 4
- Views: 183
Re: Ground State Electron Configuration
For example, if 1s22s12p3 is the excited state, 1s22s22p2 would be the ground state.
- Sun Oct 25, 2020 11:24 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation and Negative Signs
- Replies: 11
- Views: 1864
Re: Rydberg Equation and Negative Signs
If an electron jumps from a high state of energy to a low energy state, the change in energy is negative because this process release energy. However, when we use the energy later to calculate either the frequency or the wavelength, we usually do not include the negative sign, or you can understand ...
- Sun Oct 25, 2020 11:14 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: When would 3d orbital be filled before the 4s orbital?
- Replies: 8
- Views: 527
Re: When would 3d orbital be filled before the 4s orbital?
It would go as 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
- Sun Oct 25, 2020 11:09 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E.25
- Replies: 3
- Views: 316
Re: 1E.25
I think Jordan Young's answer is correct. To make an example, we would write [Ar]3d104s1 for Cu instead of 1s22s22p63s23p63d104s1
- Sun Oct 25, 2020 11:00 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Sapling Question 16
- Replies: 3
- Views: 218
Re: Sapling Question 16
I think Megan Chan's answer is correct. Just to add on it, neon's electron configuration can also be represented as [He]2s22p6
- Sun Oct 25, 2020 7:16 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: 1B.25 Second Part
- Replies: 2
- Views: 114
Re: 1B.25 Second Part
Because it's one dimensional, there will only be lines. Imagine that there is only an x-axis. The box will only have a line segment that represents its diameter.
- Sun Oct 18, 2020 4:45 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation and Empirical Equation
- Replies: 4
- Views: 238
Re: Rydberg Equation and Empirical Equation
Yes, the Rydberg Equation works for Hydrogen only, because it is based on Bohr's model which is specifically about the electrons in hydrogen atoms. There is a more complex equation 1/λ = RZ 2 ((1/n 1 2 ) - (1/n 2 2 )) that can be used for other one-electron (meaning one electron affected by nuclear ...
- Sun Oct 18, 2020 4:31 pm
- Forum: Properties of Light
- Topic: Example during lecture 6
- Replies: 5
- Views: 290
Re: Example during lecture 6
If an electron jumps from a high state of energy to a low energy state, the change in energy is negative because this process release energy. However, when we use the energy later to calculate either the frequency or the wavelength, we usually do not include the negative sign, or you can understand ...
- Sun Oct 18, 2020 9:08 am
- Forum: Photoelectric Effect
- Topic: Question about Short Wavelengths vs. Long Wavelengths
- Replies: 6
- Views: 461
Re: Question about Short Wavelengths vs. Long Wavelengths
What Ashley said is correct. In short, energy per photon needs to be great enough so that it can "take away" an electron. Therefore, if a light cannot eject electrons, that means per photon energy is not great enough. Lower wavelength means greater energy. If a light with lower wavelength ...
- Sun Oct 18, 2020 8:46 am
- Forum: Properties of Light
- Topic: energy level transfer
- Replies: 4
- Views: 192
Re: energy level transfer
So our eyes work like this: after light hits the retina, photoreceptors turn the light into electrical signals, and our brain tells us that "there is light". Therefore, when at excited state, there is no photon emitted, which means there is no light hitting the retina. Therefore, it's not ...
- Sun Oct 18, 2020 5:28 am
- Forum: Properties of Light
- Topic: Need help on 1.16
- Replies: 4
- Views: 2848
Re: Need help on 1.16
1/λ = RH (1/n12 - 1/n22)
1/λ × 1/RH = (1/n12 - 1/n22)
(1/n12 - 1/n22) = 1/(4.34 × 10-7) × 1/(1.096776 × 107) = 0.21
0.21 = 1/4 - 1/25
4 = 22, 25 = 52
Therefore, n2 = 5, n1 = 2
1/λ × 1/RH = (1/n12 - 1/n22)
(1/n12 - 1/n22) = 1/(4.34 × 10-7) × 1/(1.096776 × 107) = 0.21
0.21 = 1/4 - 1/25
4 = 22, 25 = 52
Therefore, n2 = 5, n1 = 2
- Fri Oct 09, 2020 9:04 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Confidence Interval and Accuracy & Precision
- Replies: 5
- Views: 319
Re: Confidence Interval and Accuracy & Precision
Victor, that's a novel way of thinking about confidence intervals and I definitely never had that thought occur to me. It took me a couple tries, but I think I understood what you're trying to get at in general. which makes the former more precise while the latter more accurate I'm confused about t...

