Search found 112 matches
- Sun Mar 14, 2021 11:36 am
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 557335
Re: Saying Thank You to Dr. Lavelle
Dear Dr. Lavelle, all the TA's, UA's, and staff who helped make online school possible, Thank you for all of your hard work! With everything going on during this pandemic, I was anxious about being unable to learn or do well in the course. However, you all went above and beyond during these last two...
- Sun Mar 14, 2021 11:28 am
- Forum: Student Social/Study Group
- Topic: Planning on dorming in the Fall?
- Replies: 61
- Views: 3536
Re: Planning on dorming in the Fall?
I hope to get an apartment with my friends! Fingers crossed that COVID gets better :))
- Fri Mar 12, 2021 11:49 am
- Forum: Student Social/Study Group
- Topic: How are y'all doing?
- Replies: 46
- Views: 2695
Re: How are y'all doing?
This is the most unmotivated I've ever been. It honestly worries me, but this quarter is ending soon, so there is some light at the end of the tunnel! Hopefully we all can have a fresh, strong start with Spring quarter. I also feel like things are looking up with COVID, so hopefully we can all go on...
- Fri Mar 12, 2021 11:38 am
- Forum: Student Social/Study Group
- Topic: It is possible to study for the final in one day?
- Replies: 44
- Views: 4225
Re: It is possible to study for the final in one day?
I'm not too sure why, but it's really relieving to know that others are in the same position as me, so thank you for this post and all the supportive comments. I plan on spending all of Friday night and Saturday studying. I think I'm going to first go through all the notes for a unit on the syllabus...
- Fri Mar 12, 2021 11:33 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp Units
- Replies: 5
- Views: 452
Kp Units
Hello! I'm studying for the final and I recall someone mentioning that you're only supposed to plug in pressure values in the bar unit in a Kp expression. If someone could verify this for me or further explain why that is, that'd be much appreciated! Thank you
- Fri Mar 12, 2021 10:02 am
- Forum: Student Social/Study Group
- Topic: Classes for next quarter?
- Replies: 165
- Views: 16350
Re: Classes for next quarter?
I'll be starting the LS7 series, LS40, and a cluster class!
- Wed Mar 10, 2021 8:18 am
- Forum: First Order Reactions
- Topic: Sapling Week 9/10 #11, Part 2 [ENDORSED]
- Replies: 3
- Views: 300
Sapling Week 9/10 #11, Part 2 [ENDORSED]
Hello! I can't seem to figure out this problem and I'd really appreciate it if someone could let me know if I'm setting my equation wrong. Given: [A]knot = 0.0530 mols/L, t=3.9 hours, k = 3.7×10−5 s−1 I plugged it into the first order reaction like so: A=0.0530 mol/L * e^-(3.7*10^-5 s^-1)(14040 s) =...
- Tue Mar 02, 2021 5:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Problem 6.57
- Replies: 2
- Views: 266
Textbook Problem 6.57
Hello! For this problem, the solutions manual had listed the following reactions as the anode/cathode. However, when I did it, I reversed them because I thought that since the first equation had a smaller Ereduction value, it would be oxidized and thus be the anode. If someone could help me with thi...
- Mon Mar 01, 2021 12:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Problem 6M.7
- Replies: 4
- Views: 266
Textbook Problem 6M.7
Hello! For this problem, I'm confused about which half reaction and standard potential value you would use to order these elements. For example, for Cr, there's a half reaction with Cr3+ --> Cr2+, Cr3+ --> Cr(s), and Cr2+ --> Cr(s), just to name a few. Thanks for the help!
- Sat Feb 27, 2021 7:20 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling Week 7/8 #17
- Replies: 3
- Views: 297
Re: Sapling Week 7/8 #17
I'm just as confused as you are; I used the same equation for my question and got it right, so I can't find anything wrong with your numbers. When I plugged it into my calculator I got the same answer as you. Have you tried inputting it as -0.007446? No I didn't. I put in -0.0068 and that worked. T...
- Sat Feb 27, 2021 9:44 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling Week 7/8 #17
- Replies: 3
- Views: 297
Sapling Week 7/8 #17
Hello! So I'm really struggling with my problem and I keep on getting the same answer that Sapling says is wrong. I plugged in values into Nernst equation like so:
E = E(knot) - 0.0592V/n * log(Q) = 0 - 0.0592V/2 * log (1.2/(0.82)^2 = -0.00744 V
Thank you for your help!
E = E(knot) - 0.0592V/n * log(Q) = 0 - 0.0592V/2 * log (1.2/(0.82)^2 = -0.00744 V
Thank you for your help!
- Fri Feb 26, 2021 10:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling #9, Week 7/8
- Replies: 8
- Views: 516
Re: Sapling #9, Week 7/8
I think I followed the process you guys described. I had calculated Ecell = 0.34 - (-0.44) = 0.78. However, Sapling said my answer is wrong. Can someone help me figure out what I'm doing wrong? Thank you!
- Fri Feb 26, 2021 12:13 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #5
- Replies: 3
- Views: 262
Re: Sapling Week 7/8 #5
Oh yeah, here's the given equation. They also tell us that the reaction takes place in a basic solution.
- Fri Feb 26, 2021 12:11 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #5
- Replies: 3
- Views: 262
Sapling Week 7/8 #5
Hi! Can someone guide me in the right direction? I'm not too sure what I'm missing or getting wrong in my answer here. Thank you!
- Fri Feb 26, 2021 12:01 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling HW Week 8 Q4
- Replies: 5
- Views: 362
Re: Sapling HW Week 8 Q4
Hi! So I actually solved this problem without taking out the Cl- and this made more sense to me. I sent my work below. Hope this helps!
- Thu Feb 25, 2021 5:17 pm
- Forum: Balancing Redox Reactions
- Topic: Week 7/8 Sapling #4
- Replies: 3
- Views: 283
Re: Week 7/8 Sapling #4
Thank you so much for your response! I would include the HCl in the half-reaction of the Au right? Since that's the element that's part of the complex ion?
- Thu Feb 25, 2021 5:06 pm
- Forum: Balancing Redox Reactions
- Topic: Week 7/8 Sapling #4
- Replies: 3
- Views: 283
Week 7/8 Sapling #4
Hello! So I figured that the HCl in the problem is there to supply the Cl- ions in the reaction, not as an oxidizing/reducing agent. In that case, would I just disregard the HCl when I balance the redox reaction? Or do I still include it? Thank you!
- Thu Feb 25, 2021 4:34 pm
- Forum: Balancing Redox Reactions
- Topic: Week 7/8 Sapling #3
- Replies: 2
- Views: 234
Week 7/8 Sapling #3
Hi all! How would I balance the half reactions in this case? The hint says to make sure to balance the O, H, and electrons in each half reactions and I'm especially confused about where the H's would go. Thanks for the help!
- Sun Feb 21, 2021 2:07 pm
- Forum: Student Social/Study Group
- Topic: Classes for next quarter?
- Replies: 165
- Views: 16350
Re: Classes for next quarter?
I had planned on taking chem 14 c next quarter, but the class filled up very quickly :/ I think I'll be taking LS 7a in place of that!
- Sun Feb 21, 2021 2:04 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm 2 De-stressing
- Replies: 92
- Views: 7314
Re: Post Midterm 2 De-stressing
I decided to get a burger and watch my favorite movies haha
- Wed Feb 17, 2021 10:11 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Focus 4 Exercise 15
- Replies: 5
- Views: 694
Re: Focus 4 Exercise 15
I think I'm most confused by the deltaHrxn here, especially those segment of the calculations: 2mol(- 167.16 kJ/mol). Where do you get the 2 from? And why do you multiply it by what I'm assuming is the deltaHf of Cl-? Thank you!
- Tue Feb 16, 2021 11:55 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy
- Replies: 3
- Views: 264
Re: Internal Energy
\Delta U = q + w and w = -P\Delta V , but the volume is constant in the calorimeter, so w = 0. q = -C_{cal}\Delta T , where \Delta T = 5.37K. C_{cal} can be found with the first reaction, where q_{reac}=-C_{cal}\Delta T , so C_{cal} = -\frac{q_{reac}}{\Delta T} , where q = -1.96 kJ, and \Delta T = ...
- Tue Feb 16, 2021 4:28 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2: Thermochemistry Outline
- Replies: 4
- Views: 401
Midterm 2: Thermochemistry Outline
Hi all! Just to confirm, midterm 2 will only go from bullet point "Explain the meanings of heat capacity and specific heat capacity" and below? Thanks!
- Sun Feb 14, 2021 7:41 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: delta U and delta H
- Replies: 8
- Views: 384
Re: delta U and delta H
Hi! The comments above me said it perfectly haha. I'd just like to add that delta U only equals delta H when a system has constant pressure and constant volume.
- Sun Feb 14, 2021 7:19 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Sapling Week 5/6 #20
- Replies: 2
- Views: 183
Sapling Week 5/6 #20
Hello! For this problem, would the point where Q=K be considered nonspontaneous? Thank you!
- Sun Feb 14, 2021 7:12 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Sapling #5 week 5 and 6
- Replies: 13
- Views: 666
Re: Sapling #5 week 5 and 6
Hi! All these answers are super helpful, so thank you all. When calculating the mols of gas in the PV=nRT equation, which temperature would you use when they give you and initial and final temperature? Thank you!
- Sun Feb 14, 2021 7:06 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Sapling Week 5/6 #5
- Replies: 7
- Views: 396
Re: Sapling Week 5/6 #5
You are not solving for mols correctly, I also did this incorrectly to start and found .4068 to be the molar heat capacity. To solve for molar heat capacity using PV=nRT, you need to use 298.25 K as the temperature since we're solving for initial mols. using this to solve for n (.0986*18=n*.0826*29...
- Sun Feb 14, 2021 7:01 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Signs of the system's ΔH and ΔS. [ENDORSED]
- Replies: 1
- Views: 62
Signs of the system's ΔH and ΔS. [ENDORSED]
Hello! On sapling week 5/6 #3, we determined phase changes based on the signs of these two state functions and they both had either negative signs or positive signs in all of the answers. In general, is there ever an instance of a system that has both a negative enthalpy and positive entropy, or vic...
- Sun Feb 07, 2021 12:28 pm
- Forum: Student Social/Study Group
- Topic: Best kdrama?
- Replies: 30
- Views: 1982
Re: Best kdrama?
I don't really watch K-dramas, but there's one that I LOVEEE so much. It's called Suspicious Partner and I honestly think it has the full package (romance, drama, comedy, all that good stuff!).
- Sun Feb 07, 2021 12:19 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Positive and Negative q Value
- Replies: 19
- Views: 13395
Positive and Negative q Value
Hello! If someone could clarify when q should be negative or positive, I'd really appreciate it. Thank you!
- Sun Feb 07, 2021 12:00 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: What equation to use when finding work
- Replies: 2
- Views: 173
Re: What equation to use when finding work
I believe you'd use W = - P x ∆V for irreversible expansions (pressure is constant). And on the other hand, you'd use W = -nRT ln (V2/V1) for reversible expansions (pressure is not constant).
- Sun Feb 07, 2021 11:55 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Sapling Week 3/4 #10
- Replies: 4
- Views: 156
Sapling Week 3/4 #10
Hello! I happen to really be struggling with this problem, specifically with the given standard enthalpy of fusion of water. Do I just add convert the grams of ice to mols and use the standard enthalpy to convert the mols to Joules? If that thinking is correct, then what am I supposed to do with thi...
- Fri Feb 05, 2021 9:50 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Week 3/4 Sapling #9
- Replies: 13
- Views: 749
Re: Week 3/4 Sapling #9
If you combine 230.0 mL of water at 25.00 C and 100.0 mL of water at 95.00 C, what is the final temperature of the mixture? Use 1.00 g/mL as the density of water. What equation should we be using to solve this problem? Thank you! Hi, I started this problem with the idea that whatever heat is lost b...
- Sun Jan 31, 2021 9:44 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure affects only gas reactions?
- Replies: 23
- Views: 1880
Re: Pressure affects only gas reactions?
I just want to emphasize what the person above me is saying ^^! If pressure changes due to the addition of an inert gas (like helium or xenon gas), then Le Chatalier's principle wouldn't be applicable and the system's equilibrium won't change.
- Sun Jan 31, 2021 9:38 am
- Forum: General Science Questions
- Topic: Careless Mistakes
- Replies: 54
- Views: 3784
Re: Careless Mistakes
I totally get where you're coming from because I'm the same way haha. My strategy has been to write EVERYTHING out clearly and neatly on a paper with lots of spaces in between. When I punch in values on a calculator, I tend to double or triple check my numbers. This has helped me a lot so far. Also,...
- Thu Jan 28, 2021 3:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Textbook Problem 4E.9
- Replies: 1
- Views: 124
Textbook Problem 4E.9
Hello! So I understand how to calculate the bond enthalpies here, but I don't quite understand why a higher bond enthalpy means that a structure is more stable. If someone could clarify this for me, I'd really appreciate it! Thank you :)
- Thu Jan 28, 2021 10:35 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.21 part C
- Replies: 4
- Views: 273
Re: 4D.21 part C
Hi! So I talked to a TA about this and he said that the solution manual is probably wrong. I got the same answer as you did, so I don't think you have to worry too much!
- Thu Jan 28, 2021 3:24 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Textbook Problem 4D.15
- Replies: 3
- Views: 249
Textbook Problem 4D.15
Hello! For this problem, I set the given enthalpy values like so: -1560 - [-1300 + 2(-286)], which equals 312 kJ/mol. The answer in the back of the textbook is -312 kJ/mol, so I'm assuming that I set the enthalpy values wrong. If someone could help me with this, I'd really appreciate it! Thank you!
- Sat Jan 23, 2021 11:15 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling week 2 #4
- Replies: 3
- Views: 244
Re: Sapling week 2 #4
Hi! So I encountered the same problem as you did. However, writing out the reaction for a base really helped me out. The reaction would be: [B] + [H20] = [BH+] + [OH-] As you can see, there are no [H+] ions here. So that means when you take log(0.0015), you're finding log[OH-], which results in pOH....
- Sat Jan 23, 2021 10:56 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 Hw 5
- Replies: 6
- Views: 349
Re: Sapling Week 2 Hw 5
I agree with the comment above me! They're basically just asking for the initial concentration and since you had already solved for [B] and [BH+] (which is equal to x), all you'd have to do is add the [B] and [BH+] concentrations at equilibrium to solve for [B] initial. Hope this helps!
- Sat Jan 23, 2021 10:48 am
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling vs. Textbook Problems
- Replies: 8
- Views: 454
Re: Sapling vs. Textbook Problems
Based on what we did during the fall, I'd say exams are much more focused on textbook problems! I remember encountering problems that were really similar to the textbook problems assigned on the syllabus, so I recommend going over those before the exam. Hope this helps!
- Fri Jan 22, 2021 9:02 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: qp = delta H
- Replies: 2
- Views: 198
qp = delta H
Hello! Dr. Lavelle wrote this equation down when he was explaining enthalpy and I don't quite understand the circumstances where qp would equal delta H. If someone could explain this, I'd really appreciate it! Thank you.
- Fri Jan 22, 2021 8:57 am
- Forum: Phase Changes & Related Calculations
- Topic: H and q
- Replies: 47
- Views: 1721
Re: H and q
I believe q denotes heat, which is basically a transfer of energy in chemical reactions. On the other hand, H is enthalpy, which is a state property.
- Sat Jan 16, 2021 2:27 pm
- Forum: Student Social/Study Group
- Topic: How to relax
- Replies: 168
- Views: 26074
Re: How to relax
I really like taking a walk when it's warm and sunny outside while listening to music!
- Sat Jan 16, 2021 2:25 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Textbook Problem 5.61
- Replies: 2
- Views: 183
Textbook Problem 5.61
Hello! Can someone explain why compressing the system (for part b) has little or no effect? I had thought that compressing a system meant that you're changing the volume, which in turn affects the pressure? Thank you in advance!
- Sat Jan 16, 2021 9:47 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5I.21
- Replies: 1
- Views: 126
Textbook Problem 5I.21
Hello! So for this problem, I found x = 4.3*10^-5 and used that to find the concentrations of CO2 and CO, which were [CO2] = 8.6*10^-5 and [CO] = 4.9*10^3. According to the solutions manual, this is correct. However, using the same x value, I found the concentration of O2 as [O2] = 4.6*10^-4, while ...
- Sat Jan 16, 2021 7:38 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Conjugate Seesaw Application
- Replies: 2
- Views: 145
Re: Conjugate Seesaw Application
Yup! Your reasoning seems sounds to me :))
- Sat Jan 16, 2021 7:35 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Textbook Question 5I.11
- Replies: 4
- Views: 237
Re: Textbook Question 5I.11
Hi! So dividing a number by 0.5 is actually the same as multiplying the number by 2. So the solutions manual still ends up with a mol/L concentration. Hope this helps!
- Sun Jan 10, 2021 1:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When to use ice table
- Replies: 4
- Views: 253
Re: When to use ice table
I think most times, you're going to have to use an ice table unless the problem explicitly states the concentrations all the reactants and products at equilibrium. From what I've noticed in the Sapling homework, problems that typically just say that a reactant/product is placed in a container to rea...
- Sat Jan 09, 2021 11:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sign of x in ICE Box
- Replies: 8
- Views: 444
Sign of x in ICE Box
Hello! So for most of the problems I've encountered, either the reactants or products start off with 0 in the beginning of the reaction. However, I'm sort of confused on how to determine which side would have positive/negative x if both the reactants and products have initial values greater than 0. ...
- Sat Jan 09, 2021 10:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5H.1
- Replies: 3
- Views: 168
Re: 5H.1
I think reading this part (chapter 5H) of the textbook is super helpful! It goes over ways to manipulate chemical reactions and their K value. I'll use part c as an example. Since the problem gives you N2(g)+3 H2(g)⇌2 NH3(g) with a K value of K=41, you can multiply the entire equation by 2 to get th...
- Thu Jan 07, 2021 1:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Table 5G.2 With Different Values of K and Kc
- Replies: 1
- Views: 120
Table 5G.2 With Different Values of K and Kc
Hello! Can someone please explain to me why there are different values for some of the reactions listed in Table 5G.2? I had thought that K was the umbrella term and Kc/Kp fell under it. However, that relationship of K and Kc doesn't make sense if there are two different values in the table. If some...
- Mon Jan 04, 2021 9:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5G.9
- Replies: 2
- Views: 175
Textbook Problem 5G.9
Hello! For part c of this problem, the answer is that the ratio of O2/O3 in the two containers is different. Can someone explain why that is? Thank you!
- Sun Dec 13, 2020 8:44 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Ground State of H
- Replies: 3
- Views: 488
Ground State of H
Hello! Just to verify, when a problem says an electron goes from a certain energy level to ground state, that just means that it goes to energy level n=1 right? Thank you in advance!
- Fri Dec 11, 2020 11:31 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Textbook Question 6B.1
- Replies: 4
- Views: 255
Re: Textbook Question 6B.1
I started by assigning the variable x to [H + ]. For our new pH (pH 2 ), we would have 0.12x M as our concentration. The rest of my process went like this: 1) I wrote out the equation for pH. pH 2 = -log(0.12x) = (-log(0.12)) + (-log(x)) 2) -log(x) is the same thing as the original pH, so we can re...
- Wed Dec 09, 2020 1:47 pm
- Forum: Lewis Acids & Bases
- Topic: Textbook Problem 6A.13
- Replies: 1
- Views: 87
Textbook Problem 6A.13
Hello! This question asks to identify the following as a Lewis acid or base. a)NH3 b)BF3 c)Ag+ d)F- e)H- The solutions for this proble won't load for me (no matter how much I refresh :') ), so I was hoping that someone could go over my solutions and tell me which ones I got right or wrong. I got: a)...
- Tue Dec 08, 2020 8:33 am
- Forum: Naming
- Topic: Complex Name for [Fe(CN)6]4-
- Replies: 3
- Views: 1308
Complex Name for [Fe(CN)6]4-
Hello! Toolbox 9C.1 in the textbook says this complex is called hexacyanidoferrate(II). However, the textbook also had this complex in a problem and its answer key says it's called hexacyanoferrate(II). The difference is just the "-ido" after the cyano. Which name is correct? And could som...
- Mon Dec 07, 2020 8:50 pm
- Forum: Hybridization
- Topic: Textbook Problem 2F.17
- Replies: 2
- Views: 176
Textbook Problem 2F.17
Hello! For this problem, the compound is: CH20, with C being the central atoms and the other three atoms surrounding it. I had thought that only the C atom had a hybridization of sp2. However, the textbook says that both the C and O are sp2 hybridized. Can someone please explain why that is for me? ...
- Mon Dec 07, 2020 8:45 pm
- Forum: Hybridization
- Topic: s-character in hybridization
- Replies: 2
- Views: 100
s-character in hybridization
Hello! Can someone please explain what it means when the s-character increases/decreases in a molecule? What exactly is the s-character and how does it affect bond angles? Thank you!
- Sun Dec 06, 2020 6:33 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Linear Molecular Shape and Bond Angle of 180.5
- Replies: 1
- Views: 122
Linear Molecular Shape and Bond Angle of 180.5
Hello! I'm going over some textbook problems and encountered a molecule with a linear shape. The textbook said it had a bond angle of 180.5 degrees and I was hoping someone could explain to me the cases where the bond angles are slightly different from what is expected. Thank you in advance!
- Sun Dec 06, 2020 6:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook Problem 2E.13D
- Replies: 1
- Views: 126
Textbook Problem 2E.13D
Hello! For this problem, the compound is N20. How would you determine which atom is the central atom? Thank you!
- Sun Dec 06, 2020 5:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand definition
- Replies: 7
- Views: 488
Re: Ligand definition
Hi there! So I believe a ligand is any ion or molecule that binds to a central atom (usually a transition metal) to form a coordination complex and the molecule/ion can only form a coordination complex by donating one or more of its electron pairs. Ligands are important because they form coordinatio...
- Thu Dec 03, 2020 4:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook Problem 2E.1
- Replies: 2
- Views: 204
Textbook Problem 2E.1
Hello! For part b) of this problem, it asks to determine whether or not a molecule with a bond angle of 180 degrees must have, may have, or may not have a lone pair(s) of electrons. I thought it didn't require a lone pair. However, the back of the textbook said it may have lone pairs. In what instan...
- Wed Dec 02, 2020 12:28 pm
- Forum: Naming
- Topic: Oxidation State
- Replies: 16
- Views: 720
Oxidation State
Hello! Can someone please explain what exactly is an oxidation state? What does it mean? Thank you!
- Sun Nov 29, 2020 1:37 am
- Forum: Student Social/Study Group
- Topic: Week 8/9 Thoughts/Worries
- Replies: 66
- Views: 3678
Re: Week 8/9 Thoughts/Worries
I feel like I'm understanding the content so far, but I still don't feel very confident for the final :/
But no matter what, I'm going to study my hardest and hope for the best. Best of luck to everyone!
But no matter what, I'm going to study my hardest and hope for the best. Best of luck to everyone!
- Sun Nov 29, 2020 1:34 am
- Forum: Sigma & Pi Bonds
- Topic: pi bonds
- Replies: 16
- Views: 1210
Re: pi bonds
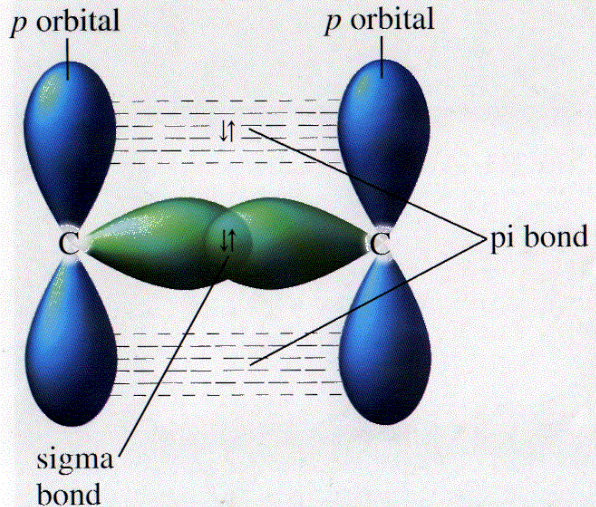

Hi! I feel like these images can help visualize the sigma/pi bonds. Hope this helps!
- Sat Nov 28, 2020 6:28 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi Bonds Being Parallel or Perpendicular
- Replies: 2
- Views: 727
Pi Bonds Being Parallel or Perpendicular
Hello! Can someone please explain to me what it means for pi bonds to be parallel or perpendicular to each other? And how do you determine when they're parallel/perpendicular? Thank you!
- Sat Nov 28, 2020 6:16 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Sapling Q.20
- Replies: 8
- Views: 1377
Sapling Q.20
Hello! The last part of this problem asked if AsO4 -3 was polar or nonpolar. I initially thought it was polar, however the solution said: "Although the bonds here are polar, the molecule is not due to resonance and the symmetrical shape of the molecule." Could someone please clarify this f...
- Sat Nov 28, 2020 6:02 pm
- Forum: Sigma & Pi Bonds
- Topic: sapling #15
- Replies: 24
- Views: 1200
Re: sapling #15
Hi! So for this problem, all you'd have to do for the sigma bonds is count how many bonds there are in total (both single and double bonds). So that would be 13 sigma bonds. Then you'd count just how many double bonds there are, so there would be 3 pi bonds.
- Sun Nov 22, 2020 3:53 pm
- Forum: Dipole Moments
- Topic: Trans-dichloroethene
- Replies: 5
- Views: 166
Re: Trans-dichloroethene
Hi! In the photo you attached, that's actually just methane, which has one carbon. Trans-dichloroethene actually has two carbons, which is why the chlorines do in fact are placed opposite from each other and cancel each other's dipole moments. Hope this helps!
- Sun Nov 22, 2020 12:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Week 7 Sapling: #5
- Replies: 4
- Views: 232
Week 7 Sapling: #5
Hello! I'm having trouble with this particular problem. What exactly are axial and equatorial atoms? Thank you!
- Wed Nov 18, 2020 6:22 am
- Forum: Lewis Structures
- Topic: N and Expanded Octet
- Replies: 3
- Views: 287
N and Expanded Octet
Hello! Can N have an expanded octet? If yes/no, why/why not?
- Tue Nov 17, 2020 8:54 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Textbook Problem 1E.5
- Replies: 3
- Views: 281
Re: Textbook Problem 1E.5
Hello! I read a portion of the textbook for some help with this problem. So s-electrons are found very close to the nucleus, which is why they're more likely to "penetrate" the nucleus, which basically just means that they may be found within the inner shells of an atoms. Since they're so ...
- Mon Nov 16, 2020 12:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Repulsion of a Bonding-Bonding Pair
- Replies: 6
- Views: 323
Repulsion of a Bonding-Bonding Pair
Hello! So in today's lecture, there was a statement that said: repulsion strength: lone-lone pair > lone-bonding pair > bonding-bonding pair What exactly does this mean? I think I'm confused because I don't quite understand the concept of a lone-bonding pair or a bonding-bonding pair. Is it the same...
- Sat Nov 14, 2020 1:35 pm
- Forum: Lewis Structures
- Topic: Nitrite vs Nitrate
- Replies: 17
- Views: 1309
Re: Nitrite vs Nitrate
I think the difference in the naming between the two is the number of O atoms in the molecule. It's the same idea with phosphate (PO4 -3) and phosphite (PO3 3-). I honestly haven't heard Professor Lavelle mention nitrite very often, nor do I recall encountering problems that mentioned nitrite, so I ...
- Sat Nov 14, 2020 1:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Why aren't the bond angles in PCl5 maximized?
- Replies: 3
- Views: 611
Re: Why aren't the bond angles in PCl5 maximized?
Hello! So to my understanding, the bond angles in PCL5 are in fact maximized. It's just that there are more atoms attached to the central atom in PCL5 than in CH4, so there are more atoms & electron repulsion in the same space. The 90 degrees bond angle is the how the linear atoms compare to the...
- Sat Nov 14, 2020 1:09 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Oxidation Number
- Replies: 11
- Views: 593
Re: Oxidation Number
All you'd have to do is set the charge of the atoms equal to the overall charge of the compound. So it'd be like:
4(O) + (Cl) = -1
4(-2) + (Cl) = -1
-8 + (Cl) = -1
Cl = +7
4(O) + (Cl) = -1
4(-2) + (Cl) = -1
-8 + (Cl) = -1
Cl = +7
- Sat Nov 14, 2020 1:04 pm
- Forum: Resonance Structures
- Topic: Finding The Most Plausible Resonance Structure
- Replies: 3
- Views: 533
Re: Finding The Most Plausible Resonance Structure
Hello there! So for finding the most plausible structure based on formal charge, you'd have to look at charges they give you in each photo. The most stable structure would be the one(s) that result in the lowest value of formal charges throughout the molecule. So in this case, it'd be structure C b/...
- Thu Nov 12, 2020 3:37 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Memorizing Formula
- Replies: 4
- Views: 373
Re: Memorizing Formula
Hi! I do something really similar to the comment above me. I simply count the electrons that are actually touching/surrounding the atoms. With lone pairs, those are easy since you can just count the "dots" we use to represent the electrons. With bonds, I just count the end(s) of the bond(s...
- Thu Nov 12, 2020 3:20 pm
- Forum: Dipole Moments
- Topic: H-Bonds
- Replies: 14
- Views: 831
H-Bonds
Hello! So basically, do H-bonds only happen when there's another compound with an atom that has a lone pair of electrons? In general, what compounds have H-bonds, because so far, I only hear H20 as a compound with H-bonds. Thank you!
- Sun Nov 08, 2020 1:52 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Electronegativity & Formal Charge
- Replies: 3
- Views: 141
Re: Electronegativity & Formal Charge
I think it's because electronegativity is the tendency of an atom to attract a shared pair of electrons. Therefore, in a compound, it would make sense to an atom with high electronegativity to attract the electrons in a compound, thus making it more negative than the rest of the atoms in the compound.
- Sun Nov 08, 2020 1:47 pm
- Forum: Octet Exceptions
- Topic: Why are there exceptions to the octect rule?
- Replies: 2
- Views: 185
Re: Why are there exceptions to the octect rule?
Hi! I think in the case of hydrogen, helium, lithium, and boron, they simply have too few valence electrons to begin with in order to be stable at a state in which they have 8 valence electrons. Hope this helps!
- Sun Nov 08, 2020 1:41 pm
- Forum: Octet Exceptions
- Topic: Octet rule
- Replies: 9
- Views: 651
Re: Octet rule
It looks like the people above me already explained the octet rule, so I'll just mention some common elements that don't follow the octet rule. These include: - boron & aluminum, who often can form compounds in which they only have six valence electrons -hydrogen, helium, & lithium because t...
- Sun Nov 08, 2020 1:25 pm
- Forum: Lewis Structures
- Topic: Lone Pairs Question
- Replies: 22
- Views: 1791
Re: Lone Pairs Question
For lewis structures, lone pairs represent an atom's pair of electrons that don't contribute to a bond of any sort with another atom. So in other words, the lone pairs are electrons that are nonbonded.
- Fri Nov 06, 2020 12:19 pm
- Forum: Ionic & Covalent Bonds
- Topic: Greek Character on 11/6 Lecture at Around Minute 16:00
- Replies: 3
- Views: 136
Greek Character on 11/6 Lecture at Around Minute 16:00
Hello! So I'm watching today's lecture and I'm curious as to what the greek letter for the slightly negative/positive atoms in the covalent bond is? What's it called and how to you write it haha. And just to be sure, that particular letter just means that an atom is positive/negative right? Thank yo...
- Mon Nov 02, 2020 12:56 pm
- Forum: Lewis Structures
- Topic: Additional Stability
- Replies: 2
- Views: 113
Re: Additional Stability
Hi! I think it's because since the electrons can move around, they can fill up the orbitals of other atoms to complete a full octet. This would make the molecules less likely to react I believe, so that's why it'd be more stable
- Sat Oct 31, 2020 5:15 pm
- Forum: Ionic & Covalent Bonds
- Topic: Is ionic or covalent stronger?
- Replies: 31
- Views: 16691
Re: Is ionic or covalent stronger?
I think ionic bonds are stronger because there's a stronger attraction between the oppositely charged atoms. And I think since covalent bonds are just atoms sharing electron pairs, it'd take less energy to break apart covalent bonds.
- Sat Oct 31, 2020 5:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Spin States and Hund's Rule
- Replies: 8
- Views: 603
Re: Spin States and Hund's Rule
Hello! I might be wrong, but I don't think it really matters which way the electron spins first. I think Professor Lavelle and other educators tend to write that the electron spins clockwise first because the arrow signifying that tends to be written on the left first, if that makes sense. So to ans...
- Sat Oct 31, 2020 5:01 pm
- Forum: Trends in The Periodic Table
- Topic: Metalloids/Non-Metals on the Periodic Table
- Replies: 6
- Views: 1750
Metalloids/Non-Metals on the Periodic Table
Hello! Is there a way or a trick to remember what elements on the right side of the periodic table are non-metals or metalloids? I tend to get confused which ones are which. Thank you in advance!
- Thu Oct 29, 2020 7:50 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Exceptions in Electron Configurations: Filling out Half/Full d-shell
- Replies: 4
- Views: 225
Exceptions in Electron Configurations: Filling out Half/Full d-shell
Hello! So in the lecture, Professor Lavelle talked about one electron moving out of the 4s shell and filling up the remaining spot in the d-shell. I'm just curious as to why the same thing doesn't happen when there are 3 or 8 electrons in the d-shell. Is it not possible for both electrons in the 4s ...
- Tue Oct 27, 2020 1:55 pm
- Forum: DeBroglie Equation
- Topic: When to Use DeBroglie Equation vs E=hv Equation
- Replies: 5
- Views: 398
When to Use DeBroglie Equation vs E=hv Equation
Hello! So I've encountered some problems that ask me to find kinetic energy and it either gives me wavelength or frequency. I've messed up on problems because I used the E=hv equation instead of the DeBroglie equation and I wanted to know if there was a way to know when it's the right time to use ei...
- Sat Oct 24, 2020 10:23 pm
- Forum: Einstein Equation
- Topic: Textbook Problem 1A.3
- Replies: 9
- Views: 483
Textbook Problem 1A.3
Hello! So the question is: Which of the following happens when the frequency of electromagnetic radiation decreases? Explain your reasoning. (a) The speed of the radiation decreases. (b) The wavelength of the radiation decreases. (c) The extent of the change in the electrical field at a given point ...
- Fri Oct 23, 2020 10:12 am
- Forum: SI Units, Unit Conversions
- Topic: SI Conversions
- Replies: 11
- Views: 405
Re: SI Conversions
I think it's good for us to memorize how to convert from m to km and g to kg. I like to think of kilo as 10^3, which means I'd just have to move the decimal place 3 times. When going from m to km, you move it 3 places to the left. From km to m, you move it 3 places to the right. Same idea for grams ...
- Fri Oct 23, 2020 10:05 am
- Forum: Properties of Light
- Topic: Units for wavelength/frequency
- Replies: 18
- Views: 1375
Re: Units for wavelength/frequency
While we're on this topic, can someone explain what an Angstrom is and how to convert to it from meters? An Angstrom simply just another unit of measurement and is 1.0 * 10^-10 meters. To convert from meters to Angstroms, you'd have to move the decimal ten places to the right. To convert from Angst...
- Fri Oct 23, 2020 9:56 am
- Forum: General Science Questions
- Topic: When to use sig figs
- Replies: 19
- Views: 815
Re: When to use sig figs
During my discussion section, my TA applied sig figs once we were finished with our calculations. I think the answer is more precise that way.
- Fri Oct 23, 2020 9:52 am
- Forum: *Shrodinger Equation
- Topic: Schrodinger Equation
- Replies: 2
- Views: 100
Re: Schrodinger Equation
Hey there! If I remember correctly, you don't have to worry too much about this equation for this course. But to answer your question, I think the Schrodinger Equation is a mathematical expression that describes the energy and position of an electron.
- Mon Oct 19, 2020 12:47 pm
- Forum: DeBroglie Equation
- Topic: E=pc Equation
- Replies: 3
- Views: 223
E=pc Equation
Hello! I'm a little confused about some of the equations Professor Lavelle wrote on his whiteboard during the lecture at minute 38:35. From what I understand, p(momentum)=m(mass)*v(velocity). Below that equation he had on the board, Professor Lavelle wrote "E=pc." I'm confused about this b...
- Sat Oct 17, 2020 7:22 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Photon Frequency and Energy Between Ground States
- Replies: 3
- Views: 127
Photon Frequency and Energy Between Ground States
Hello! Sorry if this is a dumb question, but in the lecture on 10/14 about Atomic Spectra & Energy Levels, Professor Lavelle emphasizes that an electron needs a photon that matches the energy difference between ground states in order for the electron to move from one to the other. My question is...
- Fri Oct 16, 2020 12:24 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Petition to Bring Music Back To Lectures [ENDORSED]
- Replies: 34
- Views: 1390
Re: Petition to Bring Music Back To Lectures [ENDORSED]
Was that Jack Johnson? For the song of the lecture on 10/14 ?? What song was it haha
- Fri Oct 16, 2020 8:00 am
- Forum: Photoelectric Effect
- Topic: Using variables in our work
- Replies: 8
- Views: 244
Re: Using variables in our work
Hey there! I think using h would be sufficient in your work, since it's a lot neater and less of a hassle if you use it. Just don't forget to plug in the number in your final calculation and you should be all set!

