Also, remember daylight savings changes the time!!!!
Does anyone know how many questions there will be in those 90 minutes?
Search found 112 matches
- Mon Mar 08, 2021 9:56 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Time
- Replies: 47
- Views: 2564
- Mon Mar 08, 2021 9:54 am
- Forum: Student Social/Study Group
- Topic: Review Worksheets
- Replies: 3
- Views: 382
Review Worksheets
Are there any other review worksheets like Marshmallow for Chem 14B? If so, can you send the link?
- Mon Mar 08, 2021 9:53 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Negative Change in Entropy of the Universe
- Replies: 4
- Views: 492
Re: Negative Change in Entropy of the Universe
I think for a single reaction we refer to anything not in the system as the universe or the surroundings, so for a single reaction the entropy can be negative. But, the overall trend of the universe's entropy over time will increase because it takes energy to make things more ordered but descending ...
- Mon Mar 08, 2021 9:26 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Electrochemistry
- Replies: 9
- Views: 679
Re: Electrochemistry
The nerst equation, calculating cell potential, calculating gibbs free energy in relation to E are mostly the equations we used in electrochemistry. All of them should be near each other lower on the equation sheet on Dr. Lavelle's website.
- Mon Mar 08, 2021 9:23 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: k'
- Replies: 17
- Views: 866
k'
Does k' have anything to do with the derivative of k, the rate constant, or is it just an indication of the pseudo rate constant?
- Sat Mar 06, 2021 10:01 am
- Forum: Student Social/Study Group
- Topic: Reviewing Midterm when I cant go to OH
- Replies: 5
- Views: 385
Re: Reviewing Midterm when I cant go to OH
Maybe try going asking your TA during discussion section directly considering the email option isn't working, or maybe send a followup email. Also, I think other TA's can over your exam with you as well!
- Sat Mar 06, 2021 9:57 am
- Forum: Student Social/Study Group
- Topic: Review Sessions
- Replies: 11
- Views: 1044
Re: Review Sessions
I usually try to go to each of the TA sessions (at least one for each topic). Also some of my favorite UA's to go to are Justin and Rosa. I would recommend going to as many as possible because I think that's what really helped me in 14A feel confident with the material.
- Sat Mar 06, 2021 9:53 am
- Forum: Student Social/Study Group
- Topic: 14B Final
- Replies: 22
- Views: 1074
Re: 14B Final
I think Dr. Lavelle said that the units represented on the final will be equally culumative. But, I remember on the Chem 14A final that there was slightly more of the last unit on the final than any of the other units. I wouldn't worry too much about the amount of each unit on the test, and I will b...
- Sat Mar 06, 2021 9:50 am
- Forum: Student Social/Study Group
- Topic: From pKa to pH
- Replies: 6
- Views: 451
Re: From pKa to pH
Here are the equations that I generally use:
Ka*Kb=Kw
pKa+pKb=pKw
Kw=[pOH][pH]
These principles are conceptually related to the conjugate seasaw, the idea that a stronger base will result in a weaker conjugate acid and a weaker base will result in a stronger conjugate acid and vice versa.
Ka*Kb=Kw
pKa+pKb=pKw
Kw=[pOH][pH]
These principles are conceptually related to the conjugate seasaw, the idea that a stronger base will result in a weaker conjugate acid and a weaker base will result in a stronger conjugate acid and vice versa.
- Sat Mar 06, 2021 9:45 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Dilution of Anode Concentration [ENDORSED]
- Replies: 2
- Views: 317
Re: Dilution of Anode Concentration [ENDORSED]
The concentration of the anode is always less than the concentration of the cathode. So, when you further decrease the concentration of the anode by diluting it, the difference between the concentration of the anode and the cathode is greater. Therefore, when the concentration difference is greater,...
- Sun Feb 28, 2021 4:16 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: work
- Replies: 21
- Views: 1038
Re: work
Work done on the system is positive, and work done BY the system is negative. This is because when the system is doing work it spends/loses energy to expand against exteral pressure so it would be negative. The opposite is true because energy is then inputted into the system when work is being done ...
- Sun Feb 28, 2021 4:14 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry Community Points
- Replies: 24
- Views: 1162
Re: Chemistry Community Points
Yes, you can post on Chemistry Community as much as you want. I posted around 55+ times last quarter in Chem 14A, and still received 50 points for posting. Chemistry Community is originally for learning and creating a community to answer your chemistry questions, so the more you use the greater bene...
- Sun Feb 28, 2021 4:11 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 21794
Re: Exercising Our Minds and Bodies
I have been trying to start lifting weights. I recently got a weight bench and a barbell from amazon in my basement so I've been learning the correct form for trying to deadlift and bench press.
- Sun Feb 28, 2021 4:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Drop In Sessions vs. TA OH
- Replies: 4
- Views: 707
Drop In Sessions vs. TA OH
Has anyone gone to the drop in sessions and would you say they are similar to TA office hours or the step-up sessions? Do you think it is worth it to start going to those?
- Sun Feb 28, 2021 4:04 pm
- Forum: Student Social/Study Group
- Topic: Winter GEs?
- Replies: 33
- Views: 2807
Re: Winter GEs?
I took Sociology 1 and even though the exams are a little bit difficult, I loved the content so much and it opened my eyes to social constructs that we take as fact. In addition, if you are a stem bio major like me then you will probably like taking MCDB 50- Stem Cells Ethics, Biology, and Politics....
- Fri Feb 19, 2021 3:24 pm
- Forum: Calculating Work of Expansion
- Topic: Value of Q
- Replies: 20
- Views: 1018
Re: Value of Q
Q is calculated the same way as the equilibrium constant K. So we can figure this out by taking the Q=[products]/[reactants]. If Q=K then the system is at equilibrium.
- Fri Feb 19, 2021 3:23 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm 2 De-stressing
- Replies: 92
- Views: 7634
Re: Post Midterm 2 De-stressing
Unfortunately, I have another LS7B midterm on Monday, so I will be spending my weekend studying for that. :/ But, I plan on destressing a little but by watching reruns of Dance Moms in between.
- Thu Feb 18, 2021 7:12 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Thermodynamically Favorable
- Replies: 27
- Views: 1954
Thermodynamically Favorable
What does the term thermodynamically favorable mean? Is this just in reference to the Gibbs Free Energy value or is it something else?
- Thu Feb 18, 2021 11:54 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 4J.13 Textbook Problem
- Replies: 2
- Views: 206
Re: 4J.13 Textbook Problem
Ryan_Page_1J wrote:Delta G being negative means the reaction is spontaneous, and if it is spontaneous the product will be more stable than the reactants.
So this reaction is the formation of the compounds and not the breaking down of them? Like the delta G is in reference to formation?
- Thu Feb 18, 2021 10:56 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 4J.13 Textbook Problem
- Replies: 2
- Views: 206
4J.13 Textbook Problem
4J.13 Determine which of the following compounds are stable with respect to decomposition into their elements under standard conditions at 25 C (see Appendix 2A): (a) PCl5(g); (b) HCN(g);(c) NO(g); (d) SO2(g). From my understanding, the compounds with negative Gibbs free energy would be less stable ...
- Wed Feb 17, 2021 6:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: deltaU=deltaH+w
- Replies: 8
- Views: 676
deltaU=deltaH+w
Under what circumstances can we use the delta U=delta H+ w equation? Like what allows delta H to be interchangeable with q?
- Tue Feb 09, 2021 2:50 pm
- Forum: Ideal Gases
- Topic: Chem BL
- Replies: 107
- Views: 8852
Re: Chem BL
I think I'm going to take Chem BL with Chem 14C next quarter because I read on Bruinwalk that you might need some concepts from 14B for 14BL, but that might not be true. I think Chem BL isn't too much to handle with 14C, hopefully.
- Tue Feb 09, 2021 2:47 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: lecture 14 question
- Replies: 6
- Views: 320
Re: lecture 14 question
In a reversible pathway, the changes in q occur little by little so in order to find the entropy value of the whole thing we would need to add up these small entropy changes to find the total change in entropy (delta S). This is different in an irreversible pathway because you wouldn't need an integ...
- Tue Feb 09, 2021 2:43 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: PΔV Question
- Replies: 4
- Views: 350
Re: PΔV Question
This is because gas has the capacity to significantly expand and be compressed. Liquids and solids on the other hand aren't affected as much by external pressure that would result in any non-negligible change in volume. For example if you exert a 1 atm amount of pressure on a solid, the volume doesn...
- Tue Feb 09, 2021 2:39 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Standard Molar Entropy
- Replies: 3
- Views: 260
Re: Standard Molar Entropy
Standard molar entropy is just the entropy for one mole of the substance. We don't use this as much because in thermodynamics we are often more concerned about the overall work, temperature, etc. than the molar quantities of a singular mole of a substance.
- Tue Feb 09, 2021 2:37 pm
- Forum: Student Social/Study Group
- Topic: Preparing for Midterm 2
- Replies: 14
- Views: 740
Re: Preparing for Midterm 2
About text anxiety, my best tip would be to breathe. I know this sounds simple, but I panicked a lot during the Chem 14A exams last quarter that hindered my ability to read the question without immediately trying to frantically solve it. My anxiety always lessens when I prepare for the exam harder a...
- Tue Feb 02, 2021 12:18 pm
- Forum: General Science Questions
- Topic: Outline for Midterm 2
- Replies: 2
- Views: 135
Outline for Midterm 2
What are the outlines/textbook problems that are going to be tested for Midterm 2? How much have we gone up to so far?
- Tue Feb 02, 2021 12:17 pm
- Forum: General Science Questions
- Topic: Midterm
- Replies: 10
- Views: 415
Re: Midterm
They should be back in the next week or two weeks because they have to check to see if the problems have no mistakes and assess the difficulty of the exam relative to our class performance. In the meantime, I wouldn't stress about.
- Tue Feb 02, 2021 12:11 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q= delta H
- Replies: 4
- Views: 135
Re: q= delta H
q= delta H is used when you are heating a gas at constant pressure.
- Tue Feb 02, 2021 12:05 pm
- Forum: Calculating Work of Expansion
- Topic: What is V1 and V2
- Replies: 12
- Views: 783
Re: What is V1 and V2
In addition is V1 being initial and V2 being final (similar to the variables used in the dilution equation M1V1=M2V2), this is useful when calculating change in volume = V2-V1 for the work equation w=-P(change in V).
- Tue Feb 02, 2021 12:00 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible vs Irreversible
- Replies: 4
- Views: 203
Re: Reversible vs Irreversible
To calculate the work we would use the formula work=-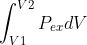
Therefore in a reversible change, when the system is in equilibrium and the external pressure is 2atm the work is different from when there is a irreversible change and the external pressure is 1atm.
Therefore in a reversible change, when the system is in equilibrium and the external pressure is 2atm the work is different from when there is a irreversible change and the external pressure is 1atm.
- Wed Jan 27, 2021 10:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4.31
- Replies: 3
- Views: 233
Re: 4.31
For part a, we can use method 3, the standard enthalpy of formation, to find the standard enthalpy of this reaction and determine if it is exothermic or endothermic. \bigtriangleup H_{RXN}^{\circ}=\sum \bigtriangleup H_{f}^{\circ} (Products) - \sum \bigtriangleup H_{f}^{\circ} (Reactant...
- Tue Jan 26, 2021 10:48 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.15
- Replies: 2
- Views: 184
4D.15
Determine the reaction enthalpy for the hydrogenation of ethyne to ethane, C2H2(g) + 2 H2(g) to C2H6(g), from the following data: delta H(C2H2, g) = -1300 kJ/mol; delta H (C2H6,g)= -1560 kJ/mol; delta H (H2,g)=-286 kJ/mol. So, I did sum or products-sum or reactants and got 312 kJ/mol, but the answer...
- Tue Jan 26, 2021 10:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Textbook question 4D.15
- Replies: 4
- Views: 274
Re: Textbook question 4D.15
Hi! Could someone explain where to start with this question? Hi Aaina, Enthalpy of a reaction is determined as the (sum of enthalpies for products) - (sum of enthalpies for reactants). Keep in mind that you should multiply the enthalpy of a substance by the number of moles they have in the reaction...
- Tue Jan 26, 2021 10:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.39 Table 5E.2
- Replies: 2
- Views: 145
Re: 5.39 Table 5E.2
Rachel Kwan 1B wrote:Do you mean Table 5G.2? I don't see Table 5E.2 mentioned in 5.39.
Haha, false alarm! Late night chem textbook problems have my eyes hallucinating I guess :)
- Tue Jan 26, 2021 9:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.39 Table 5E.2
- Replies: 2
- Views: 145
5.39 Table 5E.2
I can't seem to find Table 5E.2 mentioned in this problem 5.39 from Outline 1, can someone point to a page number or even better attach a picture of the table? Thanks!
- Mon Jan 25, 2021 9:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Table 5G.2 of Equilibrium Constants
- Replies: 1
- Views: 186
Table 5G.2 of Equilibrium Constants
How do I go about reading table 5G.2 from the textbook (i.e. what do I need to know from it)? Why are there 3 different rows of values for each reaction? Overall, I'm confused on which values to use when doing a problem.
- Mon Jan 25, 2021 4:44 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Perfect Systems
- Replies: 2
- Views: 202
Perfect Systems
Dr. Lavelle mentioned in today's lecture that a perfect system was required to use qsys+qsurr=0. Will we ever have to calculate using a non-perfect system? Should we generally assume a perfect system always?
- Mon Jan 25, 2021 4:41 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Monday's lecture clarification
- Replies: 3
- Views: 163
Re: Monday's lecture clarification
I think your confusion is in the units. -2.9 kJ= -2900 J because 1000 J equals 1 kJ. I think Dr. Lavelle said the 2900 number just as an alternative way to write your answer.
- Mon Jan 25, 2021 4:38 pm
- Forum: Phase Changes & Related Calculations
- Topic: midterm 1
- Replies: 11
- Views: 701
Re: midterm 1
Lavelle sent out an email saying that the only problems that we had to do from this week's Sapling to prepare for the exam are questions 4-8. The exam does only cover week 1-3 content only.
- Mon Jan 25, 2021 3:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive Property versus Intensive Property
- Replies: 5
- Views: 302
Re: Extensive Property versus Intensive Property
Extensive properties (like mass and volume) depend on the amount of a substance, while intensive properties (like density) are more universal and applicable because they don't depend on the amount of matter present. Here, and extensive property is less useful because we have to know the amount of su...
- Mon Jan 25, 2021 3:20 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Halogens
- Replies: 1
- Views: 119
Re: Halogens
Halogens exist in many different states of matter. Fluorine and Chlorine are the only gases (Br, I, and At are not) and I assume that they would expand similarly to any other element. I do believe that halogens have much lower boiling and melting points because of their reactivity though.
- Tue Jan 19, 2021 8:50 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increasing pressure
- Replies: 23
- Views: 972
Re: Increasing pressure
Adding an inert gas, that doesn't react to the reactants or products in the specified reaction, doesn't change the ratio of concentration of products to reactants (Kc value) or the ratio of partial pressure of products to reactants (Kp value). Therefore, increasing the pressure in this way doesn't i...
- Tue Jan 19, 2021 8:47 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Total Pressure Significance
- Replies: 4
- Views: 253
Total Pressure Significance
What is the signficance of total pressure? Why would we use total pressure rather than just needing the pressures all of the products, for example?
- Tue Jan 19, 2021 8:44 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: State indications in Chemical Equations
- Replies: 4
- Views: 186
State indications in Chemical Equations
Are all reactants or products indicated with (l) or (s), pure liquids and solids? Do we assume they are?
- Tue Jan 19, 2021 8:32 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Relevance of equilibrium constant
- Replies: 5
- Views: 452
Re: Relevance of equilibrium constant
The equilibrium constant tells us the ratio of the concentration of products to the concentration of reactants when the reaction is in equilibrium. This is important because no matter whether the amount of the products or the amount of the reactants are changed this ratio remains the same and the re...
- Tue Jan 19, 2021 8:28 am
- Forum: Administrative Questions and Class Announcements
- Topic: Lavelle's Class Website
- Replies: 11
- Views: 386
Re: Lavelle's Class Website
I haven't been having this problem. But the first time that I logged into Dr. Lavelle's website for 14B, I still had the password for 14A autosaved so it didn't work. Check to make sure that the password you are typing in is for 14A and not 14B and that you are on the right class site.
- Sun Jan 17, 2021 5:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: shifts left or right
- Replies: 23
- Views: 1172
Re: shifts left or right
If it helps, shifts left or right don't actually mean the reaction is moving. Shifts only indicate whether the reactants or products will be favored going forward.
- Sun Jan 17, 2021 5:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Carry Over
- Replies: 1
- Views: 141
Re: Sapling Carry Over
There should be an option when going to using/paying for Sapling for 14B that says "use credit", and that will mean that you don't have to pay.
- Sun Jan 17, 2021 5:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box Polynomials
- Replies: 10
- Views: 493
Re: ICE Box Polynomials
Hello, I believe that for complex polynomials like that the K value (given) is usually very insignificant, meaning less than 10^-3. As a result, the ICE box which will have for example 0.15-X the value will become simply 0.15. This is because the approximation will result in an error that is less t...
- Sun Jan 17, 2021 5:38 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: % Ionization and % Protonation
- Replies: 11
- Views: 554
% Ionization and % Protonation
I currently think about calculation of % ionization and % protonation in the same way, with the same steps. Am I right or are they different, and should I think of them differently?
- Sun Jan 17, 2021 5:32 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box Polynomials
- Replies: 10
- Views: 493
ICE Box Polynomials
For large polynomials (that can't be done with the quadratic formula) that can be difficult to solve, if there any optimum way to solve for X? I usually just graph them and find the X that way, but is there a better way or a way that Dr. Lavelle wants us to use?
- Sun Jan 17, 2021 5:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pure Liquids (Solutes)
- Replies: 7
- Views: 356
Pure Liquids (Solutes)
Other than H20, are there any other solutes of pure liquids that we should know? If we don't need to memorize it, how can you tell something will be a pure liquid or solute and insignificant to the calculations?
- Tue Jan 05, 2021 8:11 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant in terms of stability
- Replies: 4
- Views: 287
Re: Equilibrium Constant in terms of stability
From what I understood, a greater stability of the reactants means that a higher temperature is required to break them and form products. In Monday's lecture, Dr. Lavelle mentioned that he would go over temperature in relation to Kc in later lectures, so I don't think you need to know that now.
- Tue Jan 05, 2021 8:08 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry Community Quota
- Replies: 16
- Views: 1136
Re: Chemistry Community Quota
I think what the reply meant was that if you were in both Chem 14A and Chem 14B then the total # of post you should have at the end of this class would be 100 (the posts last quarter do not count this quarter essentially), but since you didn't take Chem 14A with Lavelle your total posts at the end o...
- Tue Jan 05, 2021 8:00 am
- Forum: Student Social/Study Group
- Topic: Groupme 14B Lec 2 Disc 2I
- Replies: 1
- Views: 225
Groupme 14B Lec 2 Disc 2I
Are there groupme's made for Winter 2021 Chem 14B Lec 2 or Disc 2I? If so, would anyone be willing so send the links?
- Tue Jan 05, 2021 7:53 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pure Substances
- Replies: 5
- Views: 277
Pure Substances
Dr. Lavelle mentioned in yesterday's lecture that pure substances (specifically solids and liquids) have molar concentrations that don't change in a reaction. Why are pure liquids in a reaction insignificant? I know that they are large relative to the others, but can't their change still be measured...
- Tue Jan 05, 2021 7:46 am
- Forum: Student Social/Study Group
- Topic: Advice for someone who didn't take 14A with professor Lavelle
- Replies: 61
- Views: 3125
Re: Advice for someone who didn't take 14A with professor Lavelle
To do well on the exams, it is important to watch all of his recorded lectures, do the Sapling homework on time, complete all of the textbook problems given in the outline (many of them show back up on midterms), do the audio-visual modules he provides, and if you are having trouble with a certain s...
- Mon Dec 07, 2020 1:06 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Cl- not effecting pH
- Replies: 5
- Views: 496
Re: Cl- not effecting pH
Cl- Na+ as a group 1 ion isn't strong enough to pull a hydrogen from the oxygen in water. For our purposes, as states in the textbook, group 1 and group 2 ions generally aren't strong enough.
- Mon Dec 07, 2020 1:04 pm
- Forum: Student Social/Study Group
- Topic: Recorded Lectures
- Replies: 9
- Views: 601
Re: Recorded Lectures
It was quite spotty this morning, but it should be having no problem now. I think it was a system issue and not an individualized issue.
- Mon Dec 07, 2020 12:57 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Common Ligands
- Replies: 3
- Views: 329
Common Ligands
Are we expected to know the oxidation states/numbers of common ligands like the ones listed https://sites.google.com/site/chempendix/ligands?
- Mon Dec 07, 2020 12:54 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge vs. Oxidation #
- Replies: 2
- Views: 375
Formal Charge vs. Oxidation #
What is the difference between formal charges of an atom in a compound/salt/molecule and its oxidation number?
- Mon Dec 07, 2020 12:52 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Conjugate Bases
- Replies: 3
- Views: 221
Conjugate Bases
Dr. Lavelle mentioned in today's lecture that "salts that contain the conjugate base (anion) of a weak acid will raise pH". What is a conjugate base, and why is anion in parentheses next to it?
- Mon Nov 30, 2020 8:22 am
- Forum: Administrative Questions and Class Announcements
- Topic: Finals Week
- Replies: 12
- Views: 751
Finals Week
I'm a freshman, so this is going to be my first finals week at UCLA. How does it work? Do we still have to go to classes? Is there anything I should know about it?
- Mon Nov 30, 2020 8:19 am
- Forum: Resonance Structures
- Topic: Stable Resonance Structures
- Replies: 4
- Views: 342
Re: Stable Resonance Structures
More stable resonance structures occur when the each atom (other than He or H) has a full octet and there is a small number of formal charges. For example, a -1 molecule could have a resonance structure with formal charges of -2 and +1 on their atoms. This molecule has 2 atoms with formal charge, bu...
- Mon Nov 30, 2020 8:13 am
- Forum: Dipole Moments
- Topic: Dipole arrows
- Replies: 9
- Views: 823
Re: Dipole arrows
Dipole arrows point towards the atom with partial negative charge and more electronegativity and points away from the atom with partial positive charge and less electronegativity. We can use these to solve problems when drawing things out by cancelling out arrows of dipole moments pointing in opposi...
- Mon Nov 30, 2020 8:08 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Experimental Bond Angles
- Replies: 2
- Views: 465
Re: Experimental Bond Angles
I believe that the bond angles that are fixed are ones involving no lone pairs, just bonding pairs (single, double, and/or triple). These ones we can predict by ourselves (example: tetrahedral shape with only bonding pairs is always 109.5 degrees). Lone pairs cause repulsion and the bonding pairs to...
- Mon Nov 30, 2020 7:47 am
- Forum: Coordinate Covalent Bonds
- Topic: Do C.C. bonds have expanded octet?
- Replies: 3
- Views: 252
Re: Do C.C. bonds have expanded octet?
As Dr. Lavelle mentioned in the earlier lectures, the octet rule is just a guideline. Most elements outside of the 2nd row of the periodic table can accomodate an expanded octet and don't need to follow the octet rule. The octet rule is only a strict rule for elements including carbon, nitrogen, oxy...
- Mon Nov 23, 2020 9:42 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Why are hydrogen bonds so strong relative to other dipole-dipole bonds?
- Replies: 11
- Views: 13921
Re: Why are hydrogen bonds so strong relative to other dipole-dipole bonds?
Thanks for everyone's responses! I still don't really understand why hydrogen bonds are unique in that they are so much stronger relative to other dipole-dipole bonds (-20 kJ/mol vs. -2 kJ/mol). It doesn't totally make sense to me that hydrogen bonds are 10X stronger than other dipole-dipole bonds ...
- Mon Nov 23, 2020 9:35 am
- Forum: Hybridization
- Topic: Application of Hybridized e- Configuration
- Replies: 2
- Views: 140
Application of Hybridized e- Configuration
How will we know when to use the normal electron configuration something over their hybridized electron configuration? Which contexts would we use each in?
- Mon Nov 23, 2020 9:29 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Date
- Replies: 21
- Views: 1212
Re: Final Exam Date
I believe we are taking it the date on MyUCLA because the final exam is 1 hour and 30 minutes long. This means that it would not fit within our 50 minute discussion section, unlike all of our past midterms.
- Mon Nov 23, 2020 9:23 am
- Forum: Sigma & Pi Bonds
- Topic: Sigma and Pi Bonds
- Replies: 9
- Views: 882
Re: Sigma and Pi Bonds
Sigma bonds are when two orbitals, each with one e-, are interacting END TO END. Sigma bonds allow for rotation because there is e- density symmetry around the internuclear axis (think Venn diagram shape). Pi bonds are when two orbitals, each with one e-, are interacting SIDE BY SIDE. Pi bonds can't...
- Mon Nov 23, 2020 9:16 am
- Forum: Student Social/Study Group
- Topic: Effective Studying Strategies/Resources
- Replies: 6
- Views: 371
Re: Effective Studying Strategies/Resources
I wouldn't say I've done exceptionally well, but I do believe my study strategies are fairly decent. To study, I basically do all of the practice problems in the textbook and mark which ones I got wrong and/or were unsure about. A lot of these problems are similar to the ones on the actual exam, whi...
- Wed Nov 18, 2020 10:27 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shape Names
- Replies: 50
- Views: 2590
Shape Names
What is the shape called when there are 2 lone pairs and 2 bonding pairs like H2O? Is "bent" the official shape name?
- Wed Nov 18, 2020 10:19 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Experimental Determination VSEPR
- Replies: 1
- Views: 157
Experimental Determination VSEPR
I know that VSEPR is for qualitative predictions of molecular shape and real bond angles are experimentally determined, but I'm curious how does one experimentally determine bond angles?
- Wed Nov 18, 2020 10:01 am
- Forum: Electronegativity
- Topic: Calculating elctronegativity
- Replies: 8
- Views: 458
Re: Calculating elctronegativity
Electronegativity calculations aren't something we are expected to know because usually they are given. The only thing we would need to do with them is to interpret them, recognize the periodic trend, and compare them between molecules/atoms/salts/compounds.
- Wed Nov 18, 2020 9:53 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles of SOCl2
- Replies: 1
- Views: 167
Re: Bond Angles of SOCl2
I think the bond angle is less than 180 degrees, more like around 109.5 because there is one non-bonding lone pair attached to the sulfur which would be more repulsive and make the other 3 bonding pairs be pushed down. Here, I think the shape is trigonal pyramidal similar to the example we did in le...
- Wed Nov 18, 2020 9:49 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Outline 2 - properties of electrons
- Replies: 2
- Views: 317
Re: Outline 2 - properties of electrons
In my lecture notes from October 28 (the lecture about electron configuration and the atomic properties), I have written down that s block elements have low ionization atoms and form cations. P block tends to gain electrons to form complete shells, greater electron affinity. For d- block I wrote tha...
- Thu Nov 12, 2020 9:45 am
- Forum: Lewis Structures
- Topic: Sapling Week 5-6 HW Question 6
- Replies: 7
- Views: 341
Re: Sapling Week 5-6 HW Question 6
I am having some trouble with this question as well. In the explanation for why CO2 is a Lewis acid, Sapling says that molecules with polar double bonds accept electrons, but why are the double bonds in CO2 polar? Oxygen has a far greater electronegativity compared to Carbon, so the covalent bond i...
- Tue Nov 10, 2020 9:14 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chem 14B and Chem 14BL
- Replies: 10
- Views: 629
Re: Chem 14B and Chem 14BL
I've heard that Chem 14BL would be better to take separate from Chem 14B because Chem 14BL is easier after learning the material in Chem 14B. Personally, I'm taking Chem 14C and Chem 14BL together in spring quarter.
- Tue Nov 10, 2020 9:05 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Can an Anion be Polarizing
- Replies: 4
- Views: 158
Re: Can an Anion be Polarizing
I think this is related to the charge. An cation is a positively charged ion, which means that it gives an electron to an anion. This means that the cation is polarizing because it has the ability to polarize and give it's electrons away. The anion, a negatively charged ion, is polarizable because i...
- Tue Nov 10, 2020 8:57 am
- Forum: Ionic & Covalent Bonds
- Topic: Valence Electron Count
- Replies: 3
- Views: 288
Re: Valence Electron Count
Adding to what has already been said, if the d-orbital already has 10 electrons then they aren't considered valence electrons because that shell would be full. If we remember the example in the lecture, the d orbital of the Scandium element can be described as 3d1 4s2 and not 4s2 3d1. So here, the d...
- Tue Nov 10, 2020 8:50 am
- Forum: Dipole Moments
- Topic: Dipole Moment Units
- Replies: 2
- Views: 102
Re: Dipole Moment Units
I think usually the preferred unit would be coulombs*meter, as that is the SI unit, rather than using debye. But, since our exams our multiple choice, you would never need to decide which unit to use (either would be correct).
- Tue Nov 10, 2020 8:32 am
- Forum: Ionic & Covalent Bonds
- Topic: Molecules, Compounds, and Salts
- Replies: 2
- Views: 182
Molecules, Compounds, and Salts
I remember Dr. Lavelle referring to covalently bonded elements as molecules and ionically bonded elements as compounds/salts. Does this mean compounds can't be covalently bound and molecules can't be ionically bound (are they exclusive?)?
- Mon Nov 02, 2020 2:36 pm
- Forum: Student Social/Study Group
- Topic: Participation
- Replies: 56
- Views: 3209
Re: Participation
The points aren't posted to CCLE or MyUCLA, but you can just check by yourself to see if you've posted the right amount of times by taking the week # and multiplying it by 5 and comparing it to the number of matches in the "your posts" section.
- Mon Nov 02, 2020 2:33 pm
- Forum: General Science Questions
- Topic: Midterm 2
- Replies: 4
- Views: 216
Re: Midterm 2
Midterm 2 will again be 50 minutes long, and I'm pretty sure it's multiple choice but I could be wrong.
- Mon Nov 02, 2020 1:18 pm
- Forum: Lewis Structures
- Topic: Different Lewis Structures
- Replies: 6
- Views: 349
Re: Different Lewis Structures
I believe they would be considered different resonance structures because in the example in the lecture from today Lavelle was talking about nitrate with the double bond on the left and on the right as separate structures/variations. While these are mirror images, they are considered separately.
- Mon Nov 02, 2020 8:17 am
- Forum: Lewis Structures
- Topic: Brackets for Anions
- Replies: 6
- Views: 2004
Re: Brackets for Anions
In the lecture, Dr. Lavelle mentioned that the brackets are just for clarity for the student's learning rather than being official notation, I believe.
- Mon Nov 02, 2020 7:05 am
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Week 5/6 HW
- Replies: 2
- Views: 196
Sapling Week 5/6 HW
So, this morning I clicked the link on CCLE for the Sapling HW for this week, and the website came up with the message, "Sorry, this activity is currently hidden". I was just wondering when it will open or if this is just a me problem.
- Wed Oct 28, 2020 3:10 pm
- Forum: Properties of Light
- Topic: Frequency and Wavelength
- Replies: 3
- Views: 154
Frequency and Wavelength
Just making sure, frequency and wavelength are ALWAYS positive, right?
- Mon Oct 26, 2020 3:02 pm
- Forum: Administrative Questions and Class Announcements
- Topic: sapling questions for midterm prep
- Replies: 2
- Views: 129
Re: sapling questions for midterm prep
I think all of the questions except the ones on quantum numbers and periodic trends on the Sapling assignment should be on the midterm.
- Mon Oct 26, 2020 2:57 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Dilution Problem G5 from tetxbook
- Replies: 2
- Views: 309
Re: Dilution Problem G5 from tetxbook
For part c, I would begin by changing the mg of Na2CO3 into moles of Na2CO3. This can be done by changing mg into grams by dividing by 1000 and then changing grams to moles by dividing grams by the molar mass of Na2CO3 which is 105.99 g/mol. The resulting asnwer should be around 0.000472 mol Na2CO3....
- Mon Oct 26, 2020 2:49 pm
- Forum: Ionic & Covalent Bonds
- Topic: variable valence
- Replies: 2
- Views: 256
Re: variable valence
I believe variable valency occurs when an element displays different valency (the number of valence electrons it has) when combining with various elements depending on the nature of a reaction.
You can read more about in this link: https://sciencing.com/variable-valency-11372429.html
You can read more about in this link: https://sciencing.com/variable-valency-11372429.html
- Mon Oct 26, 2020 2:41 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: How to Calculate Uncertainty
- Replies: 6
- Views: 321
Re: How to Calculate Uncertainty
Heisenberg's Uncertainty/Indeterminacy equation is (delta p)(delta x) \geq h/4 \Pi . Usually in a question, like the one done in class, they would give you either the uncertainty in position (delta x) or the uncertainty in momentum (delta p). From there you can use the equation to solve for the vari...
- Mon Oct 26, 2020 2:25 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Equation Sheet for Midterm
- Replies: 1
- Views: 143
Re: Equation Sheet for Midterm
When you go to Dr. Lavelle's website, https://lavelle.chem.ucla.edu/class-websites/chem14a/, there are tabs that say "Constants and Equations" and "Periodic Table". Dr. Lavelle sent out an email about this a couple of days ago I believe.
- Fri Oct 23, 2020 12:44 pm
- Forum: Einstein Equation
- Topic: Usage of E=hv
- Replies: 10
- Views: 533
Usage of E=hv
Does the E=hv and E=hc/wavelength equation only work for the energy of an incoming photon and not the energy of the emitted electron?
- Tue Oct 20, 2020 5:41 pm
- Forum: DeBroglie Equation
- Topic: DeBroglie Importance
- Replies: 8
- Views: 230
DeBroglie Importance
I know deBroglie suggested that the wavelength=h/p equation worked for any particle with momentum and that electrons have wavelike properties. But, why is it so important? What are the applications of knowing that electrons behave like both waves and particles?
- Tue Oct 20, 2020 5:34 pm
- Forum: Einstein Equation
- Topic: Sapling Number 4
- Replies: 6
- Views: 273
Re: Sapling Number 4
To begin, I realized that they gave us the frequency of the photons and the kinetic energy and wanted us to find the work (threshold energy) of the metal. I then decided to convert the frequency of the photons to energy of the photons by using the equation E=hv. With the energy of a photon, I used t...
- Tue Oct 20, 2020 5:21 pm
- Forum: Photoelectric Effect
- Topic: Intensity Proportional to Number of Photons?
- Replies: 4
- Views: 309
Re: Intensity Proportional to Number of Photons?
Intensity is proportional to the number of photons and can be described as the number of photons that pass a given area per second. So as the intensity increases, the # of photons increases too. In the lecture, Dr. Lavelle mentions that increasing the intensity of a light source DOES NOT increase th...
- Tue Oct 20, 2020 8:51 am
- Forum: Photoelectric Effect
- Topic: Fun with Kinetic Energy
- Replies: 2
- Views: 209
Re: Fun with Kinetic Energy
Given that the metals in both instances are the same, I know that the work of both instances will be equal. The equation for work is work=E(photon)-E(kinetic). The equation for energy of a photon can be expressed as E=ch/wavelength by combining E=hv and c=v*wavelength, and the kinetic energy is give...

