Search found 109 matches
- Tue Mar 16, 2021 12:14 am
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 376823
Re: Final Jitters
If you have the time, try to make a practice quiz for yourself, one where you put away all your notes, study guides, cheat sheets. Just solving problems on a blank piece of paper can let you know if you're ready or not depending on how confidently you can solve certain problems vs blanking out on ot...
- Tue Mar 16, 2021 12:08 am
- Forum: Student Social/Study Group
- Topic: How to relax
- Replies: 168
- Views: 28020
Re: How to relax
I like to just lay down and listen to music or take a shower to clear up my mind. Just any activity that puts your mind back at ease. Having a good laugh works as well too.
- Tue Mar 16, 2021 12:05 am
- Forum: Student Social/Study Group
- Topic: Final Study Tips!
- Replies: 38
- Views: 2345
Re: Final Study Tips!
Time yourself while doing the textbook problems!! It will make you SO much more confident for the actual test, because you'll know that you'll be able to finish it on time or with time left over! I usually give myself about 3 minutes per problem, so if a set has 7 questions, I time myself for 21 mi...
- Tue Mar 16, 2021 12:03 am
- Forum: Administrative Questions and Class Announcements
- Topic: Grading for Chemistry Community Posts
- Replies: 13
- Views: 1102
Re: Grading for Chemistry Community Posts
Is the points for Chemistry community post updated manually by the TA?
- Tue Mar 16, 2021 12:01 am
- Forum: Student Social/Study Group
- Topic: Final Motivation
- Replies: 7
- Views: 862
Re: Final Motivation
Thanks for the words of encouragement :) mindset really does matter
- Mon Mar 15, 2021 11:57 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 2
- Replies: 33
- Views: 2268
Re: Midterm 2
I also found midterm 1 more challenging as well even though the outines covered in midterm 1 was overall easier.
- Mon Mar 15, 2021 11:53 pm
- Forum: Administrative Questions and Class Announcements
- Topic: THANK YOU DR LAVELLE!
- Replies: 47
- Views: 6777
Re: THANK YOU DR LAVELLE!
Thank you Dr. Lavelle for such a wholesome two quarters! I feel very grateful to have taken general chemistry in such a positive and supportive environment you created.
PS. I really appreciated the little additions you had at the end of lectures, especially the thoughtful poetry!
PS. I really appreciated the little additions you had at the end of lectures, especially the thoughtful poetry!
- Mon Mar 15, 2021 11:46 pm
- Forum: Student Social/Study Group
- Topic: Planning on dorming in the Fall?
- Replies: 61
- Views: 3662
Re: Planning on dorming in the Fall?
If we get to go on campus and dorm, will it most likely be a double instead of the usual triple?
- Sat Mar 13, 2021 11:20 pm
- Forum: Second Order Reactions
- Topic: Molecularity
- Replies: 17
- Views: 831
Molecularity
Does the molecularity of the slow elementary step always match that of the overall rate law?
Or can differ when intermediates are involved in the reactant side of the slow step?
Or can differ when intermediates are involved in the reactant side of the slow step?
- Sat Mar 13, 2021 11:02 pm
- Forum: Calculating Work of Expansion
- Topic: when is change in internal energy 0
- Replies: 10
- Views: 1905
Re: when is change in internal energy 0
Change in internal energy is 0 when energy lost by form of work is replaced by heat from the surroundings to the system, which is the case in a reversible expansion. Delta U is equal to 0 because there is no temperature change (isothermal) during this type of expansion as delta U= 3/2nRdeltaT and de...
- Sat Mar 13, 2021 10:54 pm
- Forum: Student Social/Study Group
- Topic: Textbook Q 6M.7
- Replies: 3
- Views: 301
Re: Textbook Q 6M.7
A reducing agent is what causes the other species to get reduced while it itself gets oxidized. So you would look for the species that has the lowest standard reduction potential as it is less likely to get reduced.
- Sat Mar 13, 2021 10:46 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 6
- Views: 478
Re: Catalysts
I believe catalysts are not included as either a reactant or product of the overall rate law. We identify catalysts as those that appear in the reactant to start off the reaction and as a product on a subsequent step.
- Sat Mar 13, 2021 10:41 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G and G naught
- Replies: 46
- Views: 4661
Re: Delta G and G naught
One difference between the two is that deltaG naught isn't always equal to 0 at equilibrium (negative when K>1, positive when K<1, and 0 when K=1) while delta G is equal to 0 at equilibrium (no work done).
- Sat Mar 13, 2021 10:37 pm
- Forum: Ideal Gases
- Topic: Torr as a Unit of Pressure
- Replies: 8
- Views: 840
Re: Torr as a Unit of Pressure
To stay consistent and not get messed up with units, I would convert Torr to atm using 1atm= 760 Torr. But if you don't do this before using the ideal gas law, make sure to use the R constant 62.364 L*Torr/K*mol
- Fri Mar 12, 2021 10:31 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: Textbook Problem 6.43
- Replies: 3
- Views: 837
Re: Textbook Problem 6.43
Standard cell potentials (E ) and cell potentials (E) are intensive properties, meaning that the values do not change even when the half reaction is multiplied by a coefficient.
) and cell potentials (E) are intensive properties, meaning that the values do not change even when the half reaction is multiplied by a coefficient.
- Tue Mar 09, 2021 10:19 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7.1b [ENDORSED]
- Replies: 1
- Views: 217
7.1b [ENDORSED]
Hello, 7.1 In some reactions, two or more different products can be formed by different pathways. If the product formed by the faster reaction predominates, the reaction is considered to be under kinetic control. If the predominant product is the more thermodynamically stable, the reaction is consid...
- Mon Mar 08, 2021 4:02 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.1
- Replies: 1
- Views: 195
7D.1
How did the solutions manual derive this equation given rate constants at two different temperatures? ln(k'/k)=Ea/R(1/T- 1/T')
Why isn't it ln(k'/k)= Ea/R(1/T'-1/T)
Why isn't it ln(k'/k)= Ea/R(1/T'-1/T)
- Wed Mar 03, 2021 4:33 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook 6L5
- Replies: 3
- Views: 282
Re: Textbook 6L5
So using Pt is appropriate when there is not a solid conducting mechanism to carry the charge back up to the wires. In this case, we have one solid, I2, which can't really be used as an electrode, so we would use Pt to be those. Regarding the cell diagram itself, if you've written the half reaction...
- Wed Mar 03, 2021 4:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook 6L.5
- Replies: 2
- Views: 185
Re: Textbook 6L.5
I have the same question.. Also, if I2 is a solid, why would Pt(s) be necessary?
- Sun Feb 28, 2021 3:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Textbook 6N.1b answer key n=2?
- Replies: 1
- Views: 169
Re: Textbook 6N.1b answer key n=2?
You are correct about there being transfer of 1e-.
There was an error in the solutions manual, so the answer should be K=107.
There was an error in the solutions manual, so the answer should be K=107.
- Sun Feb 28, 2021 11:27 am
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 8 #18
- Replies: 5
- Views: 330
Sapling Week 8 #18
When we are asked to write the balanced equation for the formation of Fe2O3∙3H2O(s), we just had to balance out the coefficients after writing out the reaction in the form of Metal + O2 + H2O --> M ∙H2O. But why did we not have to find the half reactions first? If we were to write out half reactions...
- Sun Feb 28, 2021 11:11 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Sapling #17
- Replies: 8
- Views: 675
Re: Sapling #17
Yes, you would use the Nernst equation but you would use a combination of concentration values and partial pressures when solving for Q so it's a little different than what we're used to! Q = PH2/[H+]^2 Hope that helps! Why are we allowed to do pressure/concentration if the units don't match up? Is...
- Wed Feb 17, 2021 10:39 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Irreversible Expansion Entropy Change
- Replies: 2
- Views: 226
Re: Irreversible Expansion Entropy Change
Here is my work. (I made a mistake on the molar heat capacity- should be Cv instead of Cp) The only part I wasn't sure about at first was using the molar heat of the diatomic ideal gas. Since Cv=3/2R only applies to monatomic ideal gases, we can't use that. Instead for diatomic ideal gases, the rela...
- Wed Feb 17, 2021 9:07 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Textbook 5J.15
- Replies: 5
- Views: 400
Re: Textbook 5J.15
Using the Gibbs free energies of formation from appendix A only works for the solving deltaG naught at 25C because the data from the appendix is also at 25C. However, you need to do the longer method and use the appendix to solve for deltaS and delta H and then solve for deltaG naught at 150C, for ...
- Wed Feb 17, 2021 8:48 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Entropy of Monoatomic vs Diatomic gas
- Replies: 5
- Views: 1612
Re: Entropy of Monoatomic vs Diatomic gas
I believe the wording of the textbook was a bit confusing for this question. To put it in more simpler terms, let's say we had 10 atoms. One container has 10 atoms as single atoms floating around and the other container had 10 atoms into diatomic molecules (5 molecules total; 2 atoms per molecule). ...
- Wed Feb 17, 2021 8:40 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Textbook Problem 4J.13
- Replies: 2
- Views: 190
Re: Textbook Problem 4J.13
First you need to find the standard free energy of formations of the compounds. The compounds with negative free energy of formations are more thermodynamically stable compared to their elements. On the other hand the compounds with positive free energy of formation are more unstable than the eleme...
- Mon Feb 15, 2021 10:37 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Internal Energy of a Closed System
- Replies: 5
- Views: 380
Internal Energy of a Closed System
Why is the change in internal energy of a closed system a function of both heat and work (deltaU= deltaH- pdeltaV)? I thought in a closed system only heat energy is transferrable and the volume cannot change because the container is sealed? Can work still be done or can volume still change within a ...
- Sat Feb 13, 2021 4:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4.19
- Replies: 1
- Views: 104
4.19
For 4.19, what is molar kinetic energy, and why do we solve it using U= 3/2nRT equation? Calculate the molar kinetic energy (in joules per mole) of Kr(g) at 55.85°C and (b) 54.85 °C. (c) The difference between the answers to parts (a) and (b) is the energy per mole that it takes to raise the tempera...
- Sat Feb 13, 2021 11:32 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: sapling #9
- Replies: 2
- Views: 210
Re: sapling #9
In order to calculate the change in entropy of the system, you can use the equation (sum of standard molar entropy of products) - (sum of standard molar entropy of reactants) using the values linked where is says "refer to standard entropy values as needed". Next, to find the change in en...
- Sat Feb 13, 2021 11:24 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Standard molar entropy of vaporization
- Replies: 1
- Views: 117
Standard molar entropy of vaporization
For Sapling #8, it asks to calculate the standard molar entropy of vaporization at 49 degrees Celsius by using the box(three-step) method. But why is entropy of vaporization at 49 degrees called "standard" molar entropy when clearly the temperature we are asked to calculate the entropy of ...
- Fri Feb 12, 2021 5:57 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard entropy of formation vs. molar entropy [ENDORSED]
- Replies: 2
- Views: 597
Standard entropy of formation vs. molar entropy [ENDORSED]
For 4J5, the solutions manual emphasizes the difference between the calculated standard entropy of formation and the molar entropy at standard conditions that we get from the appendix. But why are they different numbers? On a similar note, for 4J15, why do we need to calculate the standard entropy o...
- Wed Feb 10, 2021 11:47 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: deltaS total
- Replies: 2
- Views: 207
deltaS total
Why is 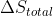 =0 or
=0 or  = -
= - in a reversible expansion?
in a reversible expansion?
- Wed Feb 10, 2021 10:50 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: HW 4I. 5
- Replies: 3
- Views: 323
Re: HW 4I. 5
I believe you would first find the final temperature of the system and then using that you would find the change in enthalpy of the hot and cold water. Then using the enthalpy, you would find the entropy of each and then combine the two entropies to find the total entropy. I initially thought of a ...
- Sun Feb 07, 2021 1:12 pm
- Forum: Student Social/Study Group
- Topic: Best kdrama?
- Replies: 30
- Views: 2029
Re: Best kdrama?
This is not a mainstream popular one, but My Mister is such a masterpiece. It may seem a bit depressing, dark, and unappealing at first, but trust me, it's one of the few dramas that really gets you emotionally. I recommend giving it a try someday.
- Sun Feb 07, 2021 1:04 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Sapling 20
- Replies: 10
- Views: 476
Re: Sapling 20
arisawaters2D wrote:is the m in this equation moles or mass
m is mass in grams
n is number of moles
We need to use q=nc
- Sun Feb 07, 2021 1:02 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Positive vs Negative Heat Capacities
- Replies: 3
- Views: 141
Re: Positive vs Negative Heat Capacities
Heat capacities should be positive because if you look at q=mc \Delta T, moles and c should always be positive so that the sign of q is dependent on change of temperature. If \Delta T is positive, q would be positive, representing the absorbance of heat. If \Delta T is negative, q would be negative,...
- Sun Feb 07, 2021 12:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Clarifying different q equations
- Replies: 1
- Views: 83
Re: Clarifying different q equations
I try to group the equations to the type of problems q is used in. For example, calorimetry, internal energy, entropy problems. For calorimetry problems, I would use q=mc \Delta T, q=nc \Delta T, qsys=-qsurr. For calculating change in internal energy, q would be used in \Delta U=q+w, \Delta U=q at c...
- Sun Feb 07, 2021 12:36 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cv vs Cp
- Replies: 2
- Views: 141
Cv vs Cp
When using q=mc \Delta T, sometimes, heat capacity at constant volume is used and sometimes heat capacity at constant pressure is used. Does that just depend on the scenario of the given problem? Also q at constant pressure is said to be equal to \Delta H . So can q only be deltaH at constant pressu...
- Sun Feb 07, 2021 12:29 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: HW 3/4 Problem 10
- Replies: 3
- Views: 256
Re: HW 3/4 Problem 10
I haven't checked the math for the final equation you got, but it seems like for the right side of the equation you are setting the initial temperature to 25 degrees. The problem says that the temp of water is 45 degrees. Maybe that can give you the correct answer.
- Sun Feb 07, 2021 12:15 pm
- Forum: Calculating Work of Expansion
- Topic: Sapling #18
- Replies: 8
- Views: 572
Re: Sapling #18
Why could we not use \Delta U =q+Pext or \Delta U= q+ \Delta nRT) to find the change in internal energy? Is it because we were not given the change in volume or the net change in moles of gas? Also, where did we get the equation \Delta U=nCv\Delta T ? I can't seem to find it in the constants and for...
- Sat Feb 06, 2021 12:42 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.3
- Replies: 2
- Views: 262
4C.3
Hello! 3. Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as heat to 0.820molKr(g) at 298 K and 1.00 atm(a) at constant pressure; (b) at constant volume. Treat the gas as ideal. The solutions manual has n(molar heat capacity at constant pressure/vol)(ch...
- Fri Feb 05, 2021 6:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Textbook Problem 4A.13
- Replies: 3
- Views: 218
Re: Textbook Problem 4A.13
Hailey Kang 2K wrote:Hi! Here's how I worked it out.
IMG_0415.jpg
Hope this helps!
Did you use a positive 3.50kJ for q because that is the amount of heat absorbed by the calorimeter from the reaction?
- Fri Feb 05, 2021 5:21 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.9
- Replies: 2
- Views: 154
4A.9
A piece of copper of mass 20.0 g at 100.0°C is placed in a vessel of negligible heat capacity but containing 50.7 g of water at 22.0°C. Calculate the final temperature of the water. Assume that no energy is lost to the surroundings. For the setup of this question, I understand that copper is losing ...
- Wed Jan 27, 2021 9:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4.31 part 2
- Replies: 2
- Views: 206
Re: 4.31 part 2
Thank you so much for the detailed explanation.
- Wed Jan 27, 2021 3:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units for Kp
- Replies: 4
- Views: 222
Units for Kp
When solving for equilibrium constant in pressure, does it matter if we use either atm or bars?
- Wed Jan 27, 2021 3:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 5I23
- Replies: 1
- Views: 108
Re: Textbook 5I23
Because the equilibrium value of CH4 is given in moles, it's easier to start off with moles so that you can let x equal 0.478 moles directly. Remember you can use any unit of measurement (moles, Molarity, pressure) for ICE tables because they're simply based on molar ratio. But if you start off with...
- Sun Jan 24, 2021 8:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4.31 part 2
- Replies: 2
- Views: 206
4.31 part 2
Water gas is an inexpensive, low-grade fuel that can be made from coal. (a) Is the production of water gas exothermic or endothermic? The reaction is C(s) +H2O(g) --> CO(g) + H2(g) (b) Calculate the enthalpy change for the production of 200. L of hydrogen at 500. Torr and 65 °C by this reaction. Hav...
- Sun Jan 24, 2021 7:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.9 bond enthalpy
- Replies: 2
- Views: 176
Re: 4E.9 bond enthalpy
I believe the solution is correct. When you calculate the total bond enthalpies based on the non-resonance structure for the distinct 3 single bonds and 3 double bonds, you get 2880 kJ. But when you calculate the bond enthalpy of the resonance structure where all 6 bonds are treated equally, you get...
- Mon Jan 18, 2021 12:28 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook problem 6D.15
- Replies: 3
- Views: 551
Re: Textbook problem 6D.15
Why did Al3+ all of a sudden become Al(H2O)6 3+?
- Sun Jan 17, 2021 9:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp to Kc conversion
- Replies: 5
- Views: 399
Kp to Kc conversion
Are we supposed to know how to convert from Kp to Kc?
We went over it during my discussion with a new formula(Kp=Kc(RT)^(difference in products and reactants coefficients)) to use for the conversion, but it wasn't in the Unit 1 outline..
We went over it during my discussion with a new formula(Kp=Kc(RT)^(difference in products and reactants coefficients)) to use for the conversion, but it wasn't in the Unit 1 outline..
- Sun Jan 17, 2021 9:02 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: When would K be unchanged?
- Replies: 31
- Views: 1212
Re: When would K be unchanged?
K increases when heat is added in an endothermic reaction, so more products form. K decreases when heat is added in an exothermic reaction, so more reactants form.
- Sun Jan 17, 2021 9:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: increasing/decreasing a solid/liquid
- Replies: 6
- Views: 312
Re: increasing/decreasing a solid/liquid
A solid doesn't have a concentration, so it would not affect the equilibrium concentrations. Liquid would not would not have an effect due to the reasons described above.
- Sun Jan 17, 2021 8:47 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Endothermic vs Exothermic
- Replies: 7
- Views: 394
Re: Endothermic vs Exothermic
As a rule of thumb, breaking a bond requires energy so it is endothermic. On the flip side, forming a bond releases energy, making it exothermic.
- Sun Jan 17, 2021 8:45 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 21280
Re: Exercising Our Minds and Bodies
I try to go on a hour walk around my neighborhood at least 2 days a week.
- Wed Jan 13, 2021 12:06 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding water to reaction
- Replies: 5
- Views: 4084
Adding water to reaction
For Textbook Problem 5.61 part f, when water was added to 6 CO2(g) + 6 H2O(l) ⇌ C6H12O6(aq) + 6 O2(g), it had no effect on the reaction because water is a liquid. The solution manual also adds that "As long as the glucose solution is dilute, its concentration can be considered unchanged". ...
- Sun Jan 10, 2021 11:25 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5.35
- Replies: 2
- Views: 113
Re: Textbook Problem 5.35
For part b of this problem (finding K), the solution manual divided the numbers by 100. For example, Partial pressure of C, instead of 10, they used (10/100). I am guessing it has something to do with the given units P/kPa. Can someone clarify the units we would normally use for partial pressure cal...
- Mon Jan 04, 2021 6:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc favoring products
- Replies: 10
- Views: 566
Kc favoring products
In the example from the lecture today, Dr. Lavelle said that a Kc of 61.0 only slightly favors products even though Kc is greater than 1. Is this because K is not larger than 10^3 that it is hard to say it strongly favors products?
- Mon Jan 04, 2021 6:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 1B post assessment #17
- Replies: 5
- Views: 221
Re: 1B post assessment #17
Why do we not have to convert bar to atm for this problem?
- Sat Dec 12, 2020 12:07 am
- Forum: Naming
- Topic: [Fe(NH3)6][Cr(CN)6]
- Replies: 1
- Views: 4370
[Fe(NH3)6][Cr(CN)6]
For [Fe(NH3)6][Cr(CN)6],the naming is Hexaammineiron (III) hexacyanochromate (III).
But how do we know what the oxidation number is if we are not given the overall charge of each complex. I know the cation and anion together have a neutral charge, but how do we know it is a 3+ and 3- charge?
But how do we know what the oxidation number is if we are not given the overall charge of each complex. I know the cation and anion together have a neutral charge, but how do we know it is a 3+ and 3- charge?
- Fri Dec 11, 2020 11:32 pm
- Forum: Hybridization
- Topic: Today's Review Video: CO2
- Replies: 2
- Views: 178
Re: Today's Review Video: CO2
Yes, I believe you are correct about (C2sp,O2sp2). To add onto that, for the pi bond between C and O, I got (C2p, O2p). And for oxygen lone pairs, the hybridization is sp2.
- Fri Dec 11, 2020 11:16 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Textbook 6B.5
- Replies: 4
- Views: 231
Re: Textbook 6B.5
You can find pH first by finding the concentration of H+ first. You can do this by using the Kw equation where [H+][OH-]=10^-14. By solving for concentration of H+, you can then plug it into -log[H+] to find the pH. Then you can find pOH by subtracting pH from 14. Hope this helps!
- Tue Dec 08, 2020 10:07 pm
- Forum: Air Pollution & Acid Rain
- Topic: acid rain
- Replies: 13
- Views: 1277
Re: acid rain
Is it CO2+water, SO2+water, or both that leads to acidic rain?
Also where in the textbook is this mentioned?
Also where in the textbook is this mentioned?
- Tue Dec 08, 2020 8:58 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Spectator Ions
- Replies: 1
- Views: 144
Spectator Ions
We were told that the conjugates of strong acids/bases won't affect the pH (for example Br- in HBr, Na+ in NaOH) because those ions are stable and the charge is not strong enough to break bonds in H2O. But why is that so? Why are those ions considered stable?
- Sun Dec 06, 2020 2:57 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 6c.19 f
- Replies: 4
- Views: 322
Re: 6c.19 f
What does it mean when high electronegativity makes an O-H bond more polar?
Does it mean that the electron density on O is shifted to a high electronegative atom that causes H to have a more partial positive charge?
Does it mean that the electron density on O is shifted to a high electronegative atom that causes H to have a more partial positive charge?
- Thu Dec 03, 2020 10:28 am
- Forum: Lewis Acids & Bases
- Topic: Lewis Acids & Bases
- Replies: 12
- Views: 1174
Re: Lewis Acids & Bases
Does this mean that lewis acids and bases don't have to always ionize in water like how Bronsted acids and bases do?
To clarify with an example, BF3 can be considered as a lewis acid just by accepting an electron pair from F- without having to dissociate anything.
To clarify with an example, BF3 can be considered as a lewis acid just by accepting an electron pair from F- without having to dissociate anything.
- Thu Dec 03, 2020 10:18 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ammonia vs Ammine
- Replies: 3
- Views: 207
Ammonia vs Ammine
I thought NH3 was ammonia, but on the list of common ligands, NH3 is named ammine.
When I searched this up on google, it said that ammine is a derivative of ammonia. What does that mean?
Is this the case because NH3 is being used as a ligand for a transition metal instead of a stand-alone compound?
When I searched this up on google, it said that ammine is a derivative of ammonia. What does that mean?
Is this the case because NH3 is being used as a ligand for a transition metal instead of a stand-alone compound?
- Mon Nov 30, 2020 5:50 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate vs Monodentate
- Replies: 10
- Views: 721
Re: Polydentate vs Monodentate
Why does the solution manual for 5b say that oxygen can bind to either one or two oxygen atoms, therefore CO3 2- can be either monodentate or bidentate?
- Mon Nov 30, 2020 5:14 pm
- Forum: Naming
- Topic: Oxidation number of ligands
- Replies: 11
- Views: 1289
Oxidation number of ligands
Are we expected to know oxidation numbers of common ligands? For example that CN has oxidation number of -1. Also do we have to memorize the list of neutral ligands along with their names?
- Sun Nov 29, 2020 10:21 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling #3
- Replies: 3
- Views: 167
Re: Sapling #3
I thought the shape is a square planar, not trigonal bipyramidal because it has a total of 6 electron densities with one as a lone pair.
- Sun Nov 29, 2020 10:18 pm
- Forum: Hybridization
- Topic: hydrogen atoms in same plane
- Replies: 4
- Views: 1050
Re: hydrogen atoms in same plane
I also had the same question. Why does an even number of C mean the hydrogens are on the same plane?
- Sun Nov 29, 2020 10:12 pm
- Forum: Electronegativity
- Topic: Be vs Cl
- Replies: 49
- Views: 2957
Re: Be vs Cl
Electronegativity is the tendency to gain an electron. Since Cl has 7 valence electrons, it is more likely to attract an electron to fulfill an octet whereas Be has 2 electrons and is more likely to lose electrons to be more stable.
- Sun Nov 29, 2020 10:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining Bond Angles
- Replies: 4
- Views: 293
Re: Determining Bond Angles
You don't have to know the exact bond angle of shapes with lone pairs. For example for trigonal pyramidal, you just have to know that the bond angles are less than 109.5. As long as you know the bond angles of basic geometry (linear, trigonal planar, tetrahedral,etc) you should be fine.
- Sun Nov 29, 2020 10:05 pm
- Forum: General Science Questions
- Topic: study methods/recs
- Replies: 37
- Views: 2429
Re: study methods/recs
Worksheets from workshops are helpful to test your knowledge on each week's topics.
The link to all worksheets can be found here https://drive.google.com/drive/folders/ ... ZvRxH_EmEc
The link to all worksheets can be found here https://drive.google.com/drive/folders/ ... ZvRxH_EmEc
- Sun Nov 29, 2020 9:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity
- Replies: 17
- Views: 729
Re: Polarity
On number 7 of Sapling HW, CH2Cl2 is polar even though C-H bonds and C-Cl bonds are directly across from each other on the Lewis structure. But the solution says that bonds aren't directly across each other on tetrahedrals. Does this mean that we always have to consider molecular shapes and not only...
- Sun Nov 29, 2020 9:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Reference for VSEPR
- Replies: 4
- Views: 382
Re: Reference for VSEPR
I'm attaching a chart that I refer to. Because each row is organized by the number of electron densities, it's easier to see everything at once.
Also this website might help you visualize the molecular shapes in 3D.
http://intro.chem.okstate.edu/1314F00/L ... VSEPR.html
Also this website might help you visualize the molecular shapes in 3D.
http://intro.chem.okstate.edu/1314F00/L ... VSEPR.html
- Sun Nov 22, 2020 10:03 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 3d and 4s
- Replies: 9
- Views: 608
Re: 3d and 4s
So does this mean that V is also an exception to the general electron configuration rules? I thought Cr and Cu were the only exceptions we needed to know.
- Sun Nov 22, 2020 9:59 pm
- Forum: Trends in The Periodic Table
- Topic: Identifying Elements
- Replies: 12
- Views: 786
Re: Identifying Elements
Groups 1 and 2 (s-block elements) are all metals because they are highly reactive due to higher likelihood of losing an electron (low electronegativity). This behavior contributes to the characteristics of metals, such as good conductor of electricity and the formation of cations. On the other hand,...
- Sun Nov 22, 2020 9:46 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: double bonding
- Replies: 8
- Views: 572
Re: double bonding
For example, water molecule H2O is bent because oxygen has two lone pairs, which push down the bond pairs between oxygen and the hydrogens due to lone pairs occupying bigger volume and the strong electron repulsion between them. As mentioned above, this molecule has bent shape due to the number of e...
- Mon Nov 16, 2020 8:37 pm
- Forum: Lewis Structures
- Topic: Identifying lewis acids and bases
- Replies: 5
- Views: 569
Re: Identifying lewis acids and bases
Will we always be given a chemical equation like the one above?
- Mon Nov 16, 2020 6:23 pm
- Forum: Bond Lengths & Energies
- Topic: IMF workshop problem
- Replies: 1
- Views: 199
IMF workshop problem
For this worksheet question, why is the boiling point of CH4O higher than CaCO3?
Based on the structures, isn't CaCO3 an ion-ion bond, which is stronger than a hydrogen bond?
If CaCO3 isn't an ion-ion bond, what intermolecular force is it, and how can you tell?
Based on the structures, isn't CaCO3 an ion-ion bond, which is stronger than a hydrogen bond?
If CaCO3 isn't an ion-ion bond, what intermolecular force is it, and how can you tell?
- Sun Nov 15, 2020 7:20 pm
- Forum: Ionic & Covalent Bonds
- Topic: Resonance Structures
- Replies: 5
- Views: 215
Re: Resonance Structures
One thing to note about hybrid resonance structures is that electrons are delocalized so all bond lengths are equal as the average of all bonds.
- Sun Nov 15, 2020 7:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling #20
- Replies: 12
- Views: 677
Re: Sapling #20
The C-H bond is no polar because there isn’t a significant electronegativity difference between C and H atoms. H atom should be bonded to a highly electronegative atom like N, O, or F in order to have a partial positive charge that allows the H atom to form a hydrogen bond with lone pairs of negativ...
- Sun Nov 08, 2020 10:29 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization and electron affinity
- Replies: 6
- Views: 462
Re: Ionization and electron affinity
Hi Joanna! IE is the energy needed to remove one electron. IE decreases down a group as the number of shells increase and the shielding effect is strengthened as the electrons of the inner shells balance out the positive charge from the nucleus, making it easier for the electron in the outer shell t...
- Sun Nov 08, 2020 10:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Pz Py Pz
- Replies: 5
- Views: 322
Re: Pz Py Pz
Px, py, pz are the 3 orbitals for the subshell p. P has 3 orbitals, and each orbital can hold 2 electrons, which have different spins.
- Mon Nov 02, 2020 10:45 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Textbook Exercise 1E.15
- Replies: 6
- Views: 187
Re: Textbook Exercise 1E.15
But why is the ground state electron configuration [Ar]3d^3 4s^2, not [Ar]3d^5? For ground state electron configuration, shouldn't all the orbitals in the lowest energy level be filled first, which in this case is 3d before 4s? For example, for question 1E.13, the ground state electron configuration...
- Mon Nov 02, 2020 10:11 pm
- Forum: Trends in The Periodic Table
- Topic: Textbook 1E.5
- Replies: 2
- Views: 192
Textbook 1E.5
This is a true-false question from the textbook. (b) Electrons in an s-orbital are more effective than those in other orbitals at shielding other electrons from the nuclear charge because an electron in an s-orbital can penetrate to the nucleus of the atom Answer: True I understand the first part of...
- Mon Nov 02, 2020 9:58 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Multielectron vs H-atom
- Replies: 4
- Views: 173
Re: Multielectron vs H-atom
So does that mean that even though the electron configuration for hydrogen is 1s1 (n=1, l=0, ml=0, ms=+1/2), the electron is not restricted to those quantum numbers and can potentially occupy n=2,3,4 shells and other sublevels? Is the electron configuration for hydrogen 1s1 simply because that is it...
- Sun Nov 01, 2020 3:21 pm
- Forum: Photoelectric Effect
- Topic: Sapling Problem #25
- Replies: 4
- Views: 228
Re: Sapling Problem #25
To find the energy of the electron, you would have to use the equation KE=1/2mv^2. In order to get the velocity, you would use the De Broglie equation, wavelength= h/(m*v). Since you're given the wavelength in micrometers, first convert that to meters, plug in value for h (6.63 x 10^-34 Js) and m (9...
- Sun Nov 01, 2020 12:23 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity
- Replies: 2
- Views: 158
Electron Affinity
For electron affinity, why does a transition to a more stable state release energy? For example, Cl releases more energy than Al by gaining an electron while He absorbs the most energy by gaining an electron? Also in the calculations we did earlier in this unit (involving \Delta E= Efinal- Einitial ...
- Sun Nov 01, 2020 11:39 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Excited vs. Ground State
- Replies: 3
- Views: 205
Excited vs. Ground State
There was a question on Sapling asking to identify the neutral element represented by the excited state electron configuration and to write a ground state electron configuration. I understand the concept of electrons being excited to a higher level, but I'm confused on how that applies to electron c...
- Sat Oct 31, 2020 5:12 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: General Heisenberg Question
- Replies: 7
- Views: 404
Re: General Heisenberg Question
I am not 100% sure, but based on most of the Heisenberg questions we practiced with, the uncertainty was expressed in actual values with units. I don't think I have seen a question with plus or minus percentages.
- Sat Oct 31, 2020 5:05 pm
- Forum: Properties of Electrons
- Topic: How to find number of electrons
- Replies: 8
- Views: 621
Re: How to find number of electrons
You would divide the total energy by the threshold energy. Because the question asks for maximum energy, you would assume that there is no excess energy, so KE=0.
- Mon Oct 26, 2020 6:26 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Textbook problem G.13
- Replies: 3
- Views: 261
Re: Textbook problem G.13
Where do you find the solution manual? Is it different from the odd-number exercise answer key on the back of the textbook?
- Sun Oct 25, 2020 2:15 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Textbook Question 1B.9
- Replies: 2
- Views: 131
Re: Textbook Question 1B.9
This is the work I did for the problem. (My sig figs are a bit off).
- Sun Oct 25, 2020 1:59 pm
- Forum: Properties of Electrons
- Topic: Marks on Midterm
- Replies: 4
- Views: 188
Re: Marks on Midterm
On ccle quizes, they usually have a column on the left with the number of points next to the question.
- Sun Oct 25, 2020 12:21 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: 1B.26
- Replies: 3
- Views: 169
1B.26
I know this problem wasn't assigned, but if anyone did it, I'd like to check answers.
What is the minimum uncertainty in the position of a hydrogen atom in a particle accelerator given that its speed is known to within +- 5.0m/s?
What is the minimum uncertainty in the position of a hydrogen atom in a particle accelerator given that its speed is known to within +- 5.0m/s?
- Sun Oct 25, 2020 11:49 am
- Forum: Balancing Chemical Reactions
- Topic: M.9 Net Ionic Equations
- Replies: 2
- Views: 2073
M.9 Net Ionic Equations
Copper(II) nitrate reacts with sodium hydroxide to produce a precipitate of light blue copper(II) hydroxide. a.) Write the net ionic equation for the reaction. b.) Calculate the maximum mass of copper(II) hydroxide that can be formed when 2.00 g of sodium hydroxide is added to 80.0 mL of 0.500M Cu(N...
- Tue Oct 20, 2020 9:29 am
- Forum: DeBroglie Equation
- Topic: Question from a step up session worksheet
- Replies: 2
- Views: 132
Question from a step up session worksheet
I have a question regarding one of the steps in the answer key for Monday's Step Up session with Melody, "Calculate the de Broglie wavelength of a 0.155 kg ball traveling at 85.0 m.s-1. Can we detect this wavelength or observe the wave-like characteristics of this ball?" I used the DeBrogl...
- Sun Oct 18, 2020 9:52 am
- Forum: Properties of Light
- Topic: Textbook 1B #3
- Replies: 3
- Views: 175
Textbook 1B #3
The question asked which effect supports the idea that electromagnetic radiation has properties of a particle, not a wave. I know that the answer is photoelectric effect because one particle of light (photon) ejects one electron, but can someone fill me in with details if I am missing any other cruc...
- Sat Oct 17, 2020 4:52 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Topic 1A #15
- Replies: 5
- Views: 222
Re: Topic 1A #15
Yes you're correct.
102.6 nm [*] (10^-9 m/ 1 nm)= 1.026 x 10^-9 m
102.6 nm [*] (10^-9 m/ 1 nm)= 1.026 x 10^-9 m

