Search found 101 matches
- Sun Mar 14, 2021 3:28 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 650755
Re: Saying Thank You to Dr. Lavelle
Dear Dr. Lavelle, Thank you so much for all the hard work and resources you put in to help us succeed these past two quarters, especially in this virtual environment! While it certainly was difficult, the opportunities that you, the TA's, and the UA's provided were invaluable and made it so much eas...
- Wed Mar 10, 2021 4:09 pm
- Forum: General Rate Laws
- Topic: rate constant of reverse reaction
- Replies: 3
- Views: 275
Re: rate constant of reverse reaction
I don't think there is a direct relation between the forward and reverse rate constant themselves. But they are related in terms of the equilibrium constant, 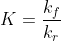
- Wed Mar 10, 2021 12:26 pm
- Forum: Calculating Work of Expansion
- Topic: Question from previous step-up
- Replies: 2
- Views: 304
Re: Question from previous step-up
Everything looks good to me!
- Tue Mar 09, 2021 11:52 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: textbook problem 6L.7 A
- Replies: 1
- Views: 131
Re: textbook problem 6L.7 A
They reversed the order because keeping it as Ag --> Ag+ would have resulted in a negative Ecell, which means the galvanic cell wouldn't be spontaneous.
- Tue Mar 09, 2021 11:48 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Sapling week 9-10. #12
- Replies: 5
- Views: 776
Re: Sapling week 9-10. #12
Given that the half-life changes when concentration changes, you can deduce that it isn't a first-order reaction. And I don't think we covered 3rd order reactions in lecture, so that should leave you with 2nd order.
- Mon Mar 08, 2021 12:28 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity and Mechanism
- Replies: 2
- Views: 233
Re: Molecularity and Mechanism
Mechanisms aren't numbers, they are the pathway through which a reaction occurs. For this course, my understanding is that we won't necessarily be finding specific mechanisms, just analyzing them.
- Fri Mar 05, 2021 3:58 pm
- Forum: General Rate Laws
- Topic: Considering Products
- Replies: 2
- Views: 191
Re: Considering Products
When working with rate laws, we look at the initial rates of the reactants (when there are no products) to keep things simple, so we won't have to worry about products in rate laws. I'm not too sure if this answered your question or not, but I hope it helps!
- Thu Mar 04, 2021 11:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Commas
- Replies: 9
- Views: 633
Re: Cell Diagram Commas
No, the order doesn't matter. Use Pt(s) when you need to add an inert conductor in the electrode (no solid metals present).
- Wed Mar 03, 2021 11:41 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: balancing charges in 6L1 part b
- Replies: 4
- Views: 356
Re: balancing charges in 6L1 part b
You'd have to look at the oxidation numbers of the compounds. The easiest one to look at is Fe. It goes from 3+ to 2+, meaning it gained an electron. And since there are 6 Fe's, 6 electrons are moved in the overall reaction, so n=6.
- Tue Mar 02, 2021 6:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagram order
- Replies: 3
- Views: 244
Re: cell diagram order
For compounds in the same phase, the order doesn't matter. For the overall cell diagram, my understanding was that you would have the inert metals like Pt on the far ends of the cell diagram and fill in the rest like:
anode reactants | anode products || cathode r | cathode p
anode reactants | anode products || cathode r | cathode p
- Mon Mar 01, 2021 11:48 am
- Forum: General Rate Laws
- Topic: When to use Differential Rate Law or Integrated Rate Law
- Replies: 3
- Views: 230
Re: When to use Differential Rate Law or Integrated Rate Law
From what I know, you can use the integrated rate law to find the concentration of a substance after some amount of time or use its graph to determine the order of the reaction. There are likely more uses for both the differential and integrated rate laws that others can comment on. But I hope this ...
- Fri Feb 26, 2021 11:22 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Undergoing oxidation/reduction
- Replies: 9
- Views: 542
Re: Undergoing oxidation/reduction
If the standard reduction potential is positive, then it's likely to be reduced. If the standard potential is negative it's likely to be oxidized.
- Thu Feb 25, 2021 11:49 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to use a comma or | in cell diagrams?
- Replies: 2
- Views: 167
When to use a comma or | in cell diagrams?
Hi everyone, I'm confused about when we should use a comma or | to separate substances when we write cell diagrams. Do we use a comma for compounds in the same phase and | when they aren't? And also when do we need to include Pt (s)?
Thanks!
Thanks!
- Wed Feb 24, 2021 11:52 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cell Pt 2
- Replies: 1
- Views: 153
Re: Concentration Cell Pt 2
From what I gathered during the lecture, I think you're correct, a concentration cell uses a concentration gradient to produce electric current. It's also helpful to use the concept of a concentration gradient to remember what happens in a concentration cell. Since particles go from high --> low con...
- Tue Feb 23, 2021 10:10 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Week 7/8 #5 terms
- Replies: 4
- Views: 336
Re: Sapling Week 7/8 #5 terms
The responses above have basically answered your question, but I just wanted to let you know that the agent is the entire compound and not just the element that is reduced or oxidized (that tripped me up when I was doing it lol). For example (this may not be correct in the problem) if Cl was oxidize...
- Mon Feb 22, 2021 10:02 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3d
- Replies: 1
- Views: 129
Re: 6K.3d
I think it may have to do with the fact that the textbook gives you a "skeletal" equation which isn't supposed to be balanced. It just shows you the compounds involved in the reaction, not necessarily the reaction itself. I may be wrong though, since I'm not too confident on skeletal equat...
- Thu Feb 18, 2021 3:21 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Michael's Workshop Cellular respiration question
- Replies: 1
- Views: 163
Re: Michael's Workshop Cellular respiration question
You ignore the products in the first step because the reaction hasn't occurred yet, so the products don't "exist." Same reasoning for the third step; the reaction has occurred and the reactants don't "exist" anymore, so you just calculate q for products and not reactants.
- Thu Feb 18, 2021 3:09 pm
- Forum: Van't Hoff Equation
- Topic: Equation Sheet
- Replies: 7
- Views: 604
Re: Equation Sheet
The second picture has a negative sign factored out, so they're technically the same.
- Wed Feb 17, 2021 11:47 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Reaction of glucose in human body
- Replies: 1
- Views: 178
Re: Reaction of glucose in human body
So the three steps are: changing temperature of reactants from 37 C to 200 C, the reaction itself at 200 C, then changing the temperature of the products from 200 C to 37 C. Calculate the \Delta H of each step, then add them all together. This would be a bit easier to explain visually, but I hope th...
- Tue Feb 16, 2021 8:55 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: w and q relationship
- Replies: 2
- Views: 202
Re: w and q relationship
- Mon Feb 15, 2021 11:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MT2 Outline 3 Content
- Replies: 3
- Views: 299
Re: MT2 Outline 3 Content
I think it should start from "Explain the meanings of heat capacity and specific heat capacity."
- Fri Feb 12, 2021 4:14 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Sapling W 5/6 #9 - clarification on standard entropy
- Replies: 2
- Views: 175
Re: Sapling W 5/6 #9 - clarification on standard entropy
I think one reason is because entropy is a state function, like enthalpy. So we can just take the entropy of the products and subtract the entropy of the reactants to get the change in entropy. \Delta S wouldn't be the same as standard entropy, since standard entropy how much entropy a substance act...
- Thu Feb 11, 2021 10:17 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Delta G and Spontaneity
- Replies: 10
- Views: 808
Re: Delta G and Spontaneity
One way to think of a spontaneous reaction is that it does not require an input of energy to proceed, so it can happen "on its own," in a sense. So energy will be released, \Delta G is negative. Since the equation for Gibbs is \Delta G=\Delta H-T\Delta S , this occurs when \Delta S is posi...
- Wed Feb 10, 2021 4:18 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Work Equation for Ideal Gas
- Replies: 5
- Views: 294
Re: Work Equation for Ideal Gas
I think the Sapling homework gives us (3/2)R and (5/2)R as the heat capacity of ideal gases in the problems, so that's probably what we can expect. It wouldn't hurt to memorize them though, they're pretty easy to remember.
- Wed Feb 10, 2021 4:09 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook 4D.7
- Replies: 2
- Views: 220
Re: Textbook 4D.7
I just assumed that if they didn't specify another temperature, to use standard conditions (25 C or 298 K)
- Mon Feb 08, 2021 11:41 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Delta H,S and Phase Changes
- Replies: 2
- Views: 154
Re: Delta H,S and Phase Changes
An increase in H will correspond to an increase in S as well (or decrease in H corresponds to decrease in S), since  for phase changes. So
for phase changes. So 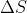 and
and  will have the same sign.
will have the same sign.
Hope this helps!
Hope this helps!
- Thu Feb 04, 2021 10:06 pm
- Forum: Phase Changes & Related Calculations
- Topic: Sapling week 3 and 4 #10
- Replies: 3
- Views: 242
Re: Sapling week 3 and 4 #10
You'll have to include the enthalpy of fusion in your calculations, to account for the heat absorbed by the ice during its phase change. Then you add that to q=mcΔt for the 0 C water, and set it equal to the negative (because it's losing heat) q=mcΔt of 45 C water.
Hope this helps!
Hope this helps!
- Thu Feb 04, 2021 12:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling Question weeks 3/4
- Replies: 1
- Views: 113
Re: Sapling Question weeks 3/4
So once you solve the first part to get the heat capacity of the calorimeter, you can use that in part 2 to calculate the heat of combustion q for compound B: 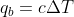 . Then you'd divide by the mass of compound B to get heat per gram.
. Then you'd divide by the mass of compound B to get heat per gram.
Hope this helps!
Hope this helps!
- Wed Feb 03, 2021 11:20 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4A. 13 Textbook problem
- Replies: 1
- Views: 68
Re: 4A. 13 Textbook problem
Since we're considering \Delta T in this problem and not the temperature itself, we can ignore the conversion between Celsius and Kelvin because \Delta T of Celsius is equal to \Delta T of Kelvin: K_{f}-K_{i}=(C_{f}+273.15)-(C_{i}+273.15)=C_{f}-C_{i} Note that you can only ignore the...
- Tue Feb 02, 2021 2:16 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Constant Volume vs. Constant Pressure
- Replies: 2
- Views: 101
Re: Constant Volume vs. Constant Pressure
At constant pressure, you'd use the equation \Delta U = q-P_{ex}\Delta V . For constant volume, you'd use the same equation except \Delta V is 0, so it would just be \Delta U = q . I'm not sure about your second question, but I think they'd tell you whether or not the pressure changes. For volume, i...
- Mon Feb 01, 2021 9:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka2 << Ka1
- Replies: 12
- Views: 1210
Re: Ka2 << Ka1
annabelchen2a wrote:Oh, is that because the Ka2 for H2SO4 is rather large/significant (in addition to its already large Ka1 value)?
Yeah, the Ka2 of H2SO4 is large enough to affect the pH so you'd include it in the calculations.
- Tue Jan 26, 2021 2:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: temperature and eq constant values
- Replies: 2
- Views: 92
Re: temperature and eq constant values
Not necessarily. It depends if the reaction is endothermic or exothermic. For exothermic, the reaction shifts to the reactants when increasing temperature, so K decreases. For endothermic, the reaction shifts towards products when increasing temperature, so K increases. The opposite is true for lowe...
- Tue Jan 26, 2021 2:43 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Textbook Q 6E 1
- Replies: 4
- Views: 216
Re: Textbook Q 6E 1
Since H2SO4 is a polyprotic acid, it'll deprotonate twice. The first time you can directly take the molarity of H+ from the molarity of H2SO4, but the second deprotonation requires the ICE table. Then you add the two values you got together and take the -log of that. Note that H2SO4 is the only poly...
- Mon Jan 25, 2021 5:49 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Difference between standard reaction enthalpy, enthalpy of formation, and enthalpy of combustion
- Replies: 2
- Views: 250
Re: Difference between standard reaction enthalpy, enthalpy of formation, and enthalpy of combustion
Standard reaction enthalpy is the enthalpy of the overall balanced reaction. Standard enthalpy of formation is the enthalpy when one mole of a substance is formed. I'm not too sure about enthalpy of combustion, but I think it's the energy released when one mole of the substance undergoes combustion....
- Mon Jan 25, 2021 5:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy vs. Standard Enthalpy of Formation
- Replies: 1
- Views: 65
Re: Standard Reaction Enthalpy vs. Standard Enthalpy of Formation
Yes, that seems about right! One other connection is that you can use standard enthalpy of formation to calculate the standard reaction enthalpy.
- Mon Jan 25, 2021 11:37 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: qsys v. qsurr
- Replies: 7
- Views: 370
Re: qsys v. qsurr
When the problem involves a calorimeter the q that you solve for is the q of the surroundings. So to find qsys you'd just take the opposite sign of that.
Hope this helps!
Hope this helps!
- Wed Jan 20, 2021 12:02 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5.61 PART B
- Replies: 3
- Views: 157
Re: 5.61 PART B
When changing the pressure, we only look at the number of moles of gas. In this case there are 6 moles of gas on each side, so there is no change.
- Wed Jan 20, 2021 11:37 am
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic v. Exothermic
- Replies: 139
- Views: 18735
Re: Endothermic v. Exothermic
I assume that's the case, since the sign of delta H is kind of "built in" to the definitions of endothermic and exothermic. Negative delta H means heat is released, so it's exothermic, while positive means heat is absorbed which is endothermic.
- Tue Jan 19, 2021 6:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Exams
- Replies: 7
- Views: 400
Re: Midterm Exams
Yeah they'll most likely be the same as last quarter where we take it in breakout rooms with TA's. And it's fine if your laptop doesn't have a camera, we used our phones for the zoom last quarter too while taking the test on computer.
- Tue Jan 19, 2021 3:01 pm
- Forum: Bronsted Acids & Bases
- Topic: Difference Between Amphoteric and Amphiprotic
- Replies: 3
- Views: 302
Re: Difference Between Amphoteric and Amphiprotic
Amphoteric compounds can act as both acids and bases. One example of an amphoteric compound is Al2O3, which can act as a Lewis acid and base despite not having any protons (H+) to donate. Amphiprotic compounds can act as both proton donors and acceptors (you can remember this by the "protic&quo...
- Tue Jan 19, 2021 2:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka2 << Ka1
- Replies: 12
- Views: 1210
Re: Ka2 << Ka1
The previous answers all explained it very well! I just wanted to add that you can ignore Ka2 for most acids except H2SO4 (this shows up in a textbook problem)
- Wed Jan 13, 2021 5:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.11 Question
- Replies: 2
- Views: 160
Re: 6B.11 Question
Using the pH, you can find the current (diluted) concentration of OH-. Then you can use M1V1=M2V2 to find the concentration of the original solution. Using the concentration you found, you can figure out the moles of NaOH, then use molar ratios and stoichiometry to find the mass of Na2O added. Hope ...
- Tue Jan 12, 2021 10:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook problem 5.57 [ENDORSED]
- Replies: 4
- Views: 448
Re: Textbook problem 5.57 [ENDORSED]
How I did it was by starting with an ICE table and filling in values for what we know, and using variables for what we don't know. Although it's a bit different for this problem since the variable isn't in the "C" row. This should leave you with one unknown variable, which you can solve fo...
- Tue Jan 12, 2021 1:28 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Approximation of X in cubic equations
- Replies: 5
- Views: 278
Re: Approximation of X in cubic equations
I'm pretty sure Kc will always be small on these questions since Dr. Lavelle said we wouldn't be able to algebraically solve cubic equations, so we have to just approximate.
- Mon Jan 11, 2021 2:02 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: ICE Table
- Replies: 9
- Views: 458
Re: ICE Table
It depends on the value of Q relative to K. If Q is greater than K, then the reaction proceeds towards the reactants, so you would put "-x" on the products side and "+x" on the reactants side. If Q is less than K, then the reaction proceeds towards products and you would put &quo...
- Mon Jan 11, 2021 1:32 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5h #3
- Replies: 5
- Views: 277
Re: Textbook Problem 5h #3
Use table 5G.2 to find two or more reactions that add together to result in the desired reaction 2BrCl(g+H2(g)⇌Br2(g)+2HCl(g). Then use the equilibrium constants for the reactions that you found in the table to calculate K for the overall reaction by multiplying them together.
Hope this helps!
Hope this helps!
- Fri Jan 08, 2021 11:35 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5G. 1
- Replies: 4
- Views: 201
Re: Textbook Problem 5G. 1
For (c), the equilibrium constant will not change due to an increase in pressure (or concentration) on one side of the reaction. Equilibrium constants for reactions only change if the temperature is changed. For (d), according to Le Chatlier's principle, if you start with a higher concentration on o...
- Thu Jan 07, 2021 11:57 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: HW problem
- Replies: 4
- Views: 243
Re: HW problem
Use table 5G.2 to find two reactions that, when added together, result in the desired reaction 2BrCl(g+H2(g)⇌Br2(g)+2HCl(g). Then you'd use the equilibrium constants for the two reactions that you found in the table to calculate K for the overall reaction by multiplying them together. Hope this helps!
- Wed Jan 06, 2021 11:30 am
- Forum: Ideal Gases
- Topic: K and PV=nRT
- Replies: 9
- Views: 968
Re: K and PV=nRT
You would use PV=nRT when you are converting from partial pressure to concentration, or vice versa. So if the problem asks for Kc, but gives you a measurement in atm, you would use PV=nRT to convert that value to the concentration and then plug it into Kc=[P]/[R]. For problems that ask for Kp, you'd...
- Tue Jan 05, 2021 12:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc vs Kp
- Replies: 14
- Views: 580
Re: Kc vs Kp
While Kc and Kp are calculated the same way, I don't think they are equal in value. Kp can be used if the reaction involves only gases, while Kc can be used for gases and aqueous solutions. The problem will usually tell you which one to solve for by looking at the units (moles for Kc, atm or bar for...
- Mon Jan 04, 2021 12:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: KC vs KP [ENDORSED]
- Replies: 6
- Views: 392
Re: KC vs KP [ENDORSED]
It would depend if we are given the values of reactants/products in concentration or bars. I remember some problems in the Audio-Visual modules had us calculate Kc when there were only gases in the reaction.
- Wed Dec 09, 2020 10:54 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation
- Replies: 2
- Views: 296
Re: Rydberg Equation
Yes, you can get the Rydberg equation by setting E=hv equal to the difference between energy levels -(hR)/n2^2 - (-(hR)/n1^2). then you cancel h from both sides to get the equation in the equation sheet. If they were asking you to calculate the energy of a specific level you'd probably use -(hR)/n^2...
- Wed Dec 09, 2020 10:47 am
- Forum: Student Social/Study Group
- Topic: lecture bruincast #29 problem
- Replies: 10
- Views: 585
Re: lecture bruincast #29 problem
I was having this problem on Monday with lecture 28, and just kept refreshing the page until it played again. It shouldn't take too long for it to work again, though.
- Wed Dec 09, 2020 10:44 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Overall Rxn K
- Replies: 2
- Views: 189
Re: Overall Rxn K
Yes, when you combine (add) the two reactions together you would multiply their individual K's together to get the K of the overall reaction. I think the equilibrium constants will be covered more in-depth in 14B, and for this class just know K A decreases as you remove more protons, and that a high...
- Tue Dec 08, 2020 11:23 am
- Forum: Lewis Acids & Bases
- Topic: general conceptual question
- Replies: 9
- Views: 841
Re: general conceptual question
I tend to first look at the bond that is being broken when an acid loses a H+, and comparing them to each other. The longer that bond is, the stronger the acid. If the bond being broken is the same across the compounds being compared, I would look at which compound has the more stable anion. There a...
- Mon Dec 07, 2020 11:06 am
- Forum: Bronsted Acids & Bases
- Topic: Acid and Base Reaction
- Replies: 1
- Views: 102
Re: Acid and Base Reaction
If the reaction involves a strong acid or base, the concentration of H3O+ or OH- will be equal to the concentration of the acid or base, respectively. If it's a weak acid or base then we need to use equilibrium which won't be tested in this course.
- Fri Dec 04, 2020 11:16 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: HClO vs. HBrO vs. HIO example
- Replies: 3
- Views: 685
Re: HClO vs. HBrO vs. HIO example
The oxygen atom is common across all three compounds, so you wouldn't use it to compare them to each other. Instead, we use the halogens because they are different in each compound and therefore comparable.
- Thu Dec 03, 2020 1:50 pm
- Forum: Naming
- Topic: Ferrate or iron?
- Replies: 8
- Views: 706
Re: Ferrate or iron?
My TA gave us some examples of when to use the Latin name for transition metals: Fe (ferrate), Cu (cuprate), Au (aurate). Looks like there's maybe a pattern here to use the Latin name when the atomic symbol isn't an exact abbreviation of its name?
- Wed Dec 02, 2020 11:12 am
- Forum: Bronsted Acids & Bases
- Topic: HCl vs. HBr
- Replies: 6
- Views: 5559
Re: HCl vs. HBr
So would it be true to say that the weaker the bond, the stronger the acid since it dissociates more easily?
- Tue Dec 01, 2020 3:08 pm
- Forum: Coordinate Covalent Bonds
- Topic: Polydentate Ligands
- Replies: 8
- Views: 522
Re: Polydentate Ligands
I'm not too sure of this myself, but I think one way to do it is to see how many atoms in the ligand have lone pairs, and use that number to determine if it is bidentate, monodentate, etc. Also consider if the ligand can rotate or not (pi bonds), since double bonds may affect how many sites a metal ...
- Mon Nov 30, 2020 10:51 am
- Forum: Naming
- Topic: Using ido or o
- Replies: 24
- Views: 1075
Re: Using ido or o
Hi! In today's lecture, Lavelle told us it was fine to use either, but he used -o in his lectures. -ido is a new naming convention that not many people know about yet.
- Fri Nov 27, 2020 3:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: TM "Octet"
- Replies: 4
- Views: 215
Re: TM "Octet"
Transition metals often don't follow the octet rule since the octet rule only considers the s and p orbitals. Transition metals are located in the d block so they often use their d-orbitals when bonding.
- Thu Nov 26, 2020 3:44 pm
- Forum: Hybridization
- Topic: Sapling #12
- Replies: 29
- Views: 1210
Re: Sapling #12
Finding the hybridization of the oxygen is similar to finding the hybridization of the carbon. You'd look at how many regions of electron density the oxygen atom has (# of bonds + lone pairs), and use that to find the hybridization.
Hope this helps!
Hope this helps!
- Wed Nov 25, 2020 11:29 am
- Forum: Biological Examples
- Topic: Why Cisplatin is considered "general"
- Replies: 4
- Views: 253
Re: Why Cisplatin is considered "general"
I'm not sure if this is what you mean by "general" or not, but it could be that cisplatin isn't specific to just cancer cells since it can also affect healthy cells. What you said about it treating many types of cancer also makes sense though!
- Tue Nov 24, 2020 10:57 am
- Forum: Administrative Questions and Class Announcements
- Topic: CHEM14B Asynchronous
- Replies: 5
- Views: 369
Re: CHEM14B Asynchronous
I think it will be, but Dr. Lavelle doesn't recommend overlapping 14B with another class since the exams might take place during the lecture time.
- Mon Nov 23, 2020 10:30 am
- Forum: Hybridization
- Topic: Unhybridized p orbital
- Replies: 2
- Views: 187
Unhybridized p orbital
Do all double bonds involve unhybridized p orbitals? What other examples of the unhybridized p orbital should we need to know?
- Thu Nov 19, 2020 12:34 am
- Forum: Lewis Structures
- Topic: NO+
- Replies: 3
- Views: 300
Re: NO+
A triple bond between the N and O is the only way for both atoms to have a full octet.
- Wed Nov 18, 2020 10:38 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Experimental Determination VSEPR
- Replies: 1
- Views: 159
Re: Experimental Determination VSEPR
I'm not sure about the specific details of how they are determined, but I remember reading somewhere that scientists can use x-ray or electron diffraction to measure bond angles in different substances.
- Tue Nov 17, 2020 10:27 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: electron spin
- Replies: 4
- Views: 281
Re: electron spin
If an orbital only has one electron and is in the ground state, I'm pretty sure the spin should be +½. It wouldn't be in the ground state if it had spin -½.
- Tue Nov 17, 2020 10:24 am
- Forum: *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Topic: Boiling and Melting point
- Replies: 24
- Views: 3151
Re: Boiling and Melting point
Boiling and melting points can be related in the sense that both are higher when the substance has stronger intermolecular forces. So yes, a higher melting (and boiling) point means stronger intermolecular forces, since more energy (heat) will be required to overcome the interactions to change phase.
- Mon Nov 16, 2020 10:50 am
- Forum: Trends in The Periodic Table
- Topic: Why don't other np4 elements behave like oxygen in terms of ionization?
- Replies: 2
- Views: 161
Re: Why don't other np4 elements behave like oxygen in terms of ionization?
Yes, I think the other Group 16 elements are also exceptions to the trend and have lower ionization energies, since they all have 4 electrons in their outermost p orbitals. This makes them have more repulsive interactions, so it takes less energy to remove an electron.
- Fri Nov 13, 2020 2:01 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: lone pairs
- Replies: 6
- Views: 382
Re: lone pairs
Lone pairs are only attracted by one nucleus, compared to a bonding pair which is attracted to two. This makes it so that lone pairs have a greater negative charge and thus more repulsion, which accounts for the extra "volume."
- Thu Nov 12, 2020 12:24 pm
- Forum: *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Topic: determining viscosity
- Replies: 11
- Views: 2694
Re: determining viscosity
From Dr. Lavelle's most recent lecture, we can determine how viscous a substance is compared to another by analyzing their IMFs. The stronger IMF's something has, the more viscous it will be.
- Wed Nov 11, 2020 10:13 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Sapling #20
- Replies: 2
- Views: 631
Re: Sapling #20
How I did it was I drew the Lewis structure for CH3CHO and used that to determine which interactions were present. I'm not sure if there's a method to determine by just using the formula, but drawing the Lewis structure should be helpful.
- Tue Nov 10, 2020 10:53 am
- Forum: Dipole Moments
- Topic: melting point
- Replies: 4
- Views: 241
Re: melting point
Yes, I believe that is the case. Since diethyl ether is nonpolar, it only has induced dipole-induced dipole interactions (LDF) between its molecules. Butanol also has LDF, but will also have dipole-dipole interactions because it is polar. So because of this extra interaction, it requires more energy...
- Mon Nov 09, 2020 11:06 am
- Forum: Octet Exceptions
- Topic: SO42- Octet Rule Exception Orbitals
- Replies: 4
- Views: 213
Re: SO42- Octet Rule Exception Orbitals
The 4s orbital is filled first before the 3d due to its lower energy. Although this isn't shown in our textbook's electron configurations, there is another way to order them called the "filling order" that shows this more clearly.


- Wed Nov 04, 2020 9:36 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Focus 1E #7b
- Replies: 2
- Views: 134
Re: Focus 1E #7b
Oh I completely missed that the second spin in 2p was pointed down. Thank you!
- Wed Nov 04, 2020 9:35 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Element orbital representation
- Replies: 4
- Views: 281
Re: Element orbital representation
In your example, the electron in the 3d orbital can act as a valence electron and determine how the element reacts, which is why it is important to include it in the noble gas configuration.
- Tue Nov 03, 2020 9:53 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Focus 1E #7b
- Replies: 2
- Views: 134
Focus 1E #7b
I'm confused about how 7b (Nitrogen) is in an excited state. I said that it was in the ground state since it has the electrons in the correct orbitals, but the answer key says otherwise.
- Mon Nov 02, 2020 10:59 am
- Forum: Lewis Structures
- Topic: Lewis Structure for NO3-
- Replies: 10
- Views: 1482
Re: Lewis Structure for NO3-
Each of the single-bonded oxygens has 3 lone pairs, which combined with the single bond gives them a full 8 electron octet. I'm not sure about the "-" in NO3-, but I hope this helps!
- Mon Nov 02, 2020 10:50 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: When to use formal charge or octet rule?
- Replies: 12
- Views: 841
When to use formal charge or octet rule?
When drawing Lewis structures, how do we know when to use the formal charge or when to use the octet rule for each element?
- Fri Oct 30, 2020 11:59 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration Shorthand Question
- Replies: 5
- Views: 314
Re: Electron Configuration Shorthand Question
Generally when shortening electron configuration, we would substitute the preceding noble gas, instead of the immediately preceding element. This allows us to see the electrons in the highest energy level (valence electrons).
- Fri Oct 30, 2020 11:57 am
- Forum: Properties of Light
- Topic: Textbook Question Number 1A 3 c
- Replies: 3
- Views: 181
Re: Textbook Question Number 1A 3 c
I saw other explanations saying that extent of change refers to amplitude. Can this also be true? I don't think so, since the amplitude corresponds to the electric field. The extent of change refers to the slope from a trough to the next crest on the wave, or vice versa. So while changing the ampli...
- Wed Oct 28, 2020 11:27 am
- Forum: DeBroglie Equation
- Topic: Brooke Yasuda's Wk 2 Workshop
- Replies: 1
- Views: 149
Re: Brooke Yasuda's Wk 2 Workshop
I think the answer key had the energy of the incident light equal to the work function because the kinetic energy of the ejected electron is so small that it's basically negligible, so adding it to the work function wouldn't change anything. I'm pretty sure either way is fine and gives the same answ...
- Tue Oct 27, 2020 11:19 am
- Forum: *Shrodinger Equation
- Topic: Particle in a Box
- Replies: 13
- Views: 662
Re: Particle in a Box
I think the amplitude of the wave is 0 at the ends of the box because that's where the box's borders are, so there is no chance that the particle will actually be at that location (since it's already taken by the edges of the box). The wavefunction represents the probability that the particle will b...
- Mon Oct 26, 2020 2:40 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic VS Molecular Spectroscopy
- Replies: 1
- Views: 131
Re: Atomic VS Molecular Spectroscopy
I'm not 100% sure, but I think there are a couple concepts in that learning outcome: one is to understand that you can analyze the spectroscopy of both atoms and molecules, and the second is to understand the differences between Lyman and Balmer series, like how they each correspond to one region an...
- Thu Oct 22, 2020 3:48 pm
- Forum: Empirical & Molecular Formulas
- Topic: Diatomic Elements
- Replies: 3
- Views: 223
Re: Diatomic Elements
I think you would use the diatomic form in calculations when the diatomic elements are alone (not bonded to anything else). Otherwise, you should use what is given in the chemical equation.
- Wed Oct 21, 2020 11:29 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Applied to particles other than electrons
- Replies: 5
- Views: 267
Re: Applied to particles other than electrons
The Heisenberg indeterminacy equation applies to all objects, but is only noticeable and mostly affects objects on an atomic scale.
- Tue Oct 20, 2020 8:41 pm
- Forum: Photoelectric Effect
- Topic: Spectral Lines
- Replies: 4
- Views: 287
Re: Spectral Lines
Spectral lines represent the wavelength of the energy absorbed or emitted by an electron when it transitions from one energy level to another. The energy absorbed or emitted is equal to the difference in energy between the two levels that the electron transitions between, and you can use E=\frac{hc}...
- Mon Oct 19, 2020 9:49 am
- Forum: Properties of Light
- Topic: How to quantify uncertainty
- Replies: 2
- Views: 147
Re: How to quantify uncertainty
The uncertainty of position or momentum is like standard deviation of either the position or momentum (velocity) of an object. For example, if an object's position was 3\pm2 m, then \Delta x would be equal to 4, as the object could be anywhere from 1 m to 5 m, and 5-1=4. Once you know the uncertaint...
- Mon Oct 19, 2020 9:34 am
- Forum: Properties of Light
- Topic: photoelectric effect module help!!
- Replies: 3
- Views: 187
Re: photoelectric effect module help!!
The answer should be D. The photoelectric effect is one of the experiments that shows the particle properties of a photon. If photons acted only like waves, then increasing the intensity (amplitude) should result in ejected electrons (ike a wave at the beach, the higher it is, the stronger it is). H...
- Thu Oct 15, 2020 10:05 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Energy gap
- Replies: 4
- Views: 140
Re: Energy gap
In addition to what Sid posted, the spectral lines in the UV region are part of the Lyman series, which has n1=1 for its lower energy level and n2=2 as its higher energy level, which is why they have higher energy and a shorter wavelength.
- Wed Oct 14, 2020 10:33 pm
- Forum: Properties of Light
- Topic: Balmer vs Lyman
- Replies: 12
- Views: 510
Re: Balmer vs Lyman
A helpful way to identify when to use each series is to remember that the Balmer series occurs within the visible light range on the EM spectrum, so it'll have a longer wavelength and less energy, which makes sense since the energy difference is smaller between the higher levels and n=2 for the Balm...
- Tue Oct 13, 2020 2:28 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Spectral Series
- Replies: 2
- Views: 248
Spectral Series
I was going over the solution to #15 in Focus 1A of the textbook, which said that each spectral series has its own lower energy level (Lyman is n=1, Balmer n=2, etc.). So this got me curious if each of the spectral series also corresponds with one region of the electromagnetic spectrum?
- Mon Oct 12, 2020 11:06 am
- Forum: Properties of Light
- Topic: c=λv
- Replies: 5
- Views: 435
Re: c=λv
While it is true that c=λv describes the relationship betwen the wavelength and frequency of light, I don't think this equation allows you to figure out the exact graph. You would be able to conclude that the shape would be oscillatory however, since wavelength and frequency are both qualities of a ...
- Mon Oct 12, 2020 10:59 am
- Forum: Properties of Light
- Topic: What exactly is Φ?
- Replies: 16
- Views: 1130
Re: What exactly is Φ?
Φ is the amount of energy (unique to each element) that a photon needs to have for an electron to be ejected from a metal when it is hit with the proton. E = hv is the energy of a photon when it hits the metal (hv = Φ + E k ). So Φ would be equal to hv only if the electron is ejected with no excess ...
- Thu Oct 08, 2020 11:45 pm
- Forum: DeBroglie Equation
- Topic: E = pc [ENDORSED]
- Replies: 2
- Views: 351
Re: E = pc [ENDORSED]
Hi there! E = pc is derived from E = mc 2 . Since c is the speed of light, we can think of E = mc 2 as E = mv 2 , or E = mvv. And since momentum p = mv, we can plug in momentum to get E = pv. After substituting c back in, we end up with E = pc. You can connect this with the equation from photoelectr...
- Wed Oct 07, 2020 3:44 pm
- Forum: Photoelectric Effect
- Topic: Which one of following is not describing the photoelectric effect?
- Replies: 3
- Views: 120
Re: Which one of following is not describing the photoelectric effect?
I also thought it was E at first, but Arielle is correct that the answer is D. My reasoning was that although λv = c is used in photoelectric effect problems, it isn't specific to the photoelectric effect itself, while the other three equations are.
Hope this helps!
Hope this helps!
- Tue Oct 06, 2020 2:23 pm
- Forum: SI Units, Unit Conversions
- Topic: Grams to Moles of Oxygen Gas
- Replies: 7
- Views: 661
Re: Grams to Moles of Oxygen Gas
Hi!
Since oxygen is one of the diatomic elements (along with Br, I, N, Cl, H, and F), it exists as O2. So when the question is talking about oxygen gas (O2), we should use 32.00 g/mol as a conversion factor.
Hope this helps!
Since oxygen is one of the diatomic elements (along with Br, I, N, Cl, H, and F), it exists as O2. So when the question is talking about oxygen gas (O2), we should use 32.00 g/mol as a conversion factor.
Hope this helps!
- Mon Oct 05, 2020 11:24 am
- Forum: Balancing Chemical Reactions
- Topic: Moles and Chemical Equations
- Replies: 12
- Views: 306
Re: Moles and Chemical Equations
Hi! The moles of each respective molecule is equal to the stoichiometric coefficient. The moles of each individual element in a molecule is equal to the stoichiometric coefficient times the subscript. So for your question, there would be 4 moles of hydrogen atoms, but 2 moles of water molecule. Hope...

