Search found 36 matches
- Sat Mar 18, 2023 2:55 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode vs Cathode
- Replies: 5
- Views: 700
Re: Anode vs Cathode
An odes are negative so it repels the electrons. Electrons leave and therefore oxidation occurs. Cat hodes are positive so it attracts the electrons. Electrons flow toward it so reduction occurs. A fun memory trick is this pun I learned. [Cat]ions are [paw]sitive. ie. Cat ions are positive so cat h...
- Sat Mar 18, 2023 2:51 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steady State
- Replies: 3
- Views: 543
Re: Steady State
Hi Minh-Anh! Steady state reaction means that the concentration of its intermediates are constant throughout the reaction.
- Sat Mar 18, 2023 1:27 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: arrhenius equation
- Replies: 3
- Views: 231
arrhenius equation
Why are there different forms of the arrhenius equation? (refer to images) And do I need to have the ln(k/k) one memorized?
- Sat Mar 18, 2023 12:49 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: - n F E
- Replies: 6
- Views: 589
- n F E
Can someone explain the relation between the wMAX and the  ? Why are both of them equal = - n F E?
? Why are both of them equal = - n F E?
- Sun Mar 12, 2023 3:20 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells Shorthand Notation
- Replies: 3
- Views: 146
Galvanic Cells Shorthand Notation
For the shorthand notation, how do I know when to put Pt(s) in the diagram and when not to? For example, why do the equations below have Pt(s) while others do not?
- Sun Mar 12, 2023 1:39 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Question 6L 3D
- Replies: 2
- Views: 119
Textbook Question 6L 3D
How do I figure out the oxidation half reaction and reduction half reaction for this?

- Sat Mar 11, 2023 2:07 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Question 6L 3B
- Replies: 1
- Views: 82
Textbook Question 6L 3B
Question asks:
(C)
How do I approach this question?
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells:
(C)
How do I approach this question?
- Sat Mar 11, 2023 1:38 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Shorthand Notation for Anode Cathode Cells
- Replies: 1
- Views: 92
Shorthand Notation for Anode Cathode Cells
For Shorthand Notation of Anode Cathode Cells how do we know the way to order the elements on the anode and cathode side?
For example
For example
Pb(s)|PbCl2(s)|Cl−(aq)‖Cl−(aq)|AgCl(s)|Ag(s)
- Sat Mar 11, 2023 1:10 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: finding -pdeltav using pv=nrt
- Replies: 4
- Views: 528
Re: finding -pdeltav using pv=nrt
For those types of questions, you should use whatever temperature given to you(this temperature could be the final or the initial depending on the situation). I am not aware of any 14B questions that give multiple temperatures.
- Sat Mar 11, 2023 12:50 am
- Forum: Balancing Redox Reactions
- Topic: 6K5.d
- Replies: 3
- Views: 136
Re: 6K5.d
Because the atoms of  split into two different compounds. Some of the P atoms become the oxidizing agents while the others become the reducing agents.
split into two different compounds. Some of the P atoms become the oxidizing agents while the others become the reducing agents.
- Sun Mar 05, 2023 11:57 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: How are ΔG, E, and K related?
- Replies: 4
- Views: 182
Re: How are ΔG, E, and K related?
\Delta G and E are related in that a spontaneous reaction means \Delta G <0 and E is >0. We can connect them by saying when one is negative, the other is positive . \Delta G and K relation can also be explained by a spontaneous reaction. In a spontaneous reaction, \Delta G <0 while K>1. This also m...
- Sun Mar 05, 2023 11:44 pm
- Forum: Balancing Redox Reactions
- Topic: How To Balance Redox
- Replies: 3
- Views: 110
Re: How To Balance Redox
There are the steps for balancing a basic redox reaction. First split them into oxidation half and reduction half reactions. Then consider the change in each element's oxidation number to determine the number of e- lost/gained. Example I will use is: X^{-}+Y+\rightarrow X+Y^{3-} Oxidation: Loss of e...
- Sun Mar 05, 2023 11:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Week 7/8 Achieve #17
- Replies: 6
- Views: 241
Re: Week 7/8 Achieve #17
To find Q take P(H2) and divide it by
because P/R and it's 2H+(aq)+2e−↽−−⇀H2(g)
For this E
- Sun Mar 05, 2023 11:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Week 7-8 Achieve HW #10
- Replies: 8
- Views: 573
Re: Week 7-8 Achieve HW #10
You need to refer to the list of elements and their reduction potentials. Higher reduction potential(likely to reduce) means better oxidizing agent.
https://sites.google.com/site/chempendix/potentials
https://sites.google.com/site/chempendix/potentials
- Mon Feb 27, 2023 12:39 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 4919739
Re: Post All Chemistry Jokes Here
This is a joke AND a memory trick:
[CAT]ions are [PAW]sitive!
[CAT]ions are [PAW]sitive!
- Mon Feb 27, 2023 12:36 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Equilibrium Equation
- Replies: 3
- Views: 949
Re: Gibbs Free Energy Equilibrium Equation
The relationship between ΔG and equilibrium is fundamental to understanding chemical reactions. At equilibrium, the forward and reverse reactions are occurring at the same rate, so there is no net change in the concentrations of the reactants and products. ΔG is related to the difference in the Gibb...
- Sun Feb 26, 2023 10:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Equilibrium Equation
- Replies: 3
- Views: 949
Re: Gibbs Free Energy Equilibrium Equation
Gibbs free energy is the potential energy. So when Gibbs free is negative, then the reaction has the potential to lose energy meaning it is spontaneous. The equation for standard Gibbs free energy is \Delta G^{o} = -RTlnK. K being greater than 1(Products>reactants at Eq) makes lnK positive, making G...
- Sun Feb 26, 2023 10:17 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Standard Cell Potential
- Replies: 4
- Views: 144
Re: Standard Cell Potential
Because adding the standard cell potentials for anodes and cathodes equal to zero.
- Sun Feb 26, 2023 10:10 pm
- Forum: Balancing Redox Reactions
- Topic: Separating a redox reaction into two half reactions
- Replies: 2
- Views: 159
Re: Separating a redox reaction into two half reactions
It is actually going from  to
to  meaning it gains electrons. How did I know that? Well...
meaning it gains electrons. How did I know that? Well...
let's say charge of Mn reactant is 'X'
Now we know the total charge of has to be -1.
has to be -1.
So now use algebra to find Mn charge (X):
-1 = X + (4*-2)
let's say charge of Mn reactant is 'X'
Now we know the total charge of
So now use algebra to find Mn charge (X):
-1 = X + (4*-2)
- Tue Feb 21, 2023 2:20 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook Question 4.15
- Replies: 1
- Views: 97
Textbook Question 4.15
In order to solve for T I must first find q. How do I find the enthalpy change for this reaction?
- Mon Feb 20, 2023 11:37 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Achieve Question 5
- Replies: 2
- Views: 122
Re: Achieve Question 5
First use the ideal gas law 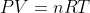
to find the number of moles(n). Then use)
to find the number of moles(n). Then use
- Mon Feb 20, 2023 11:32 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Achieve #7
- Replies: 2
- Views: 148
Re: Achieve #7
To get started, use the 4.39 minutes and take the 400W to convert to Joules. (400W=400J/s). Then find the mass of content that was vaporized, and convert that into moles. Take Joules and divide by moles.
That's how to get started but let me know if you need more explanation.
That's how to get started but let me know if you need more explanation.
- Sat Feb 18, 2023 7:01 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Reactions
- Replies: 2
- Views: 117
Reversible Reactions
Can somebody explain to me the concept of a reversible reaction, and why an infinitesmal change is possible for reversing a reaction?
- Sat Feb 11, 2023 6:56 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Achieve week 3+4 Q17
- Replies: 1
- Views: 78
Re: Achieve week 3+4 Q17
These formulas might be helpful.


- Sat Feb 11, 2023 6:46 pm
- Forum: Phase Changes & Related Calculations
- Topic: Weeks 3 and 4 Homework, #10
- Replies: 1
- Views: 115
Re: Weeks 3 and 4 Homework, #10
You have correctly calculated the heat required to melt the ice , but that value doesnt represent q. In order to find q you must combine that value to the heat required to bring 54.2g of ice from 0 degrees C to the T_{F} . Since we do not have the \Delta T , we cannot find q like that, but we can se...
- Sat Feb 11, 2023 6:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy vs. Bond Enthalpy
- Replies: 3
- Views: 97
Re: Standard Reaction Enthalpy vs. Bond Enthalpy
Bond enthalpy is the energy associated with each individual bond in a molecule or compound. Standard Reaction Enthaply is the overall energy change for the reaction as a whole. Standard reaction enthalpy can be calculated by taking the negative values bond enthalpies for each of the products(energy ...
- Sun Feb 05, 2023 12:24 am
- Forum: General Science Questions
- Topic: Week 4 Midterm
- Replies: 1
- Views: 304
Week 4 Midterm
How exactly will the midterm exam be curved?
- Sun Feb 05, 2023 12:20 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated system
- Replies: 18
- Views: 1606
Re: Isolated system
An isolated system is a system that does not intereact with its surroundings as neither matter nor energy can enter or exit it. An example could be hot water sealed tightly in a thermoflask. That way neither the water(matter) nor heat(energy) can enter or exit. In other words, it stays warm.
- Sun Feb 05, 2023 12:11 am
- Forum: Calculating Work of Expansion
- Topic: What does it mean when a reaction is reversible?
- Replies: 4
- Views: 300
Re: What does it mean when a reaction is reversible?
To add on, reversible reactions are where the K constant comes into play. K represents the ratio between products:reactants at equilibrium. Only reversible reactions will have equilibrium concentrations for reactants and products.
- Mon Jan 30, 2023 2:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Question 5.35
- Replies: 1
- Views: 129
Textbook Question 5.35
How do I go about solving part A and part B?
- Sun Jan 29, 2023 11:08 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Monoprotic acids
- Replies: 2
- Views: 186
Re: Monoprotic acids
When an acid is monoprotic it means each atom contains one and can donate one proton. When an acid is weak it means that the acid's solution will not dissociate all the way. In other words, not all the reactants will convert meaning not all the molecules will dissociate and donate. As for calculatin...
- Sun Jan 29, 2023 11:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 5I #33
- Replies: 1
- Views: 82
Re: Textbook 5I #33
Create an ICE table. To find the initial concentration(M) of ammonium carbamate([ NH_{4}(NH_{2}CO_{2}) ) take the initial mass(25.0g) and convert it to moles and divide it by the volume(0.250L). The initial concentrations of NH3 and CO2 are 0. Then for equilibrium concentration of ammonium ...
- Sun Jan 29, 2023 10:32 pm
- Forum: General Science Questions
- Topic: Chemistry Study Sessions [ENDORSED]
- Replies: 1
- Views: 240
Chemistry Study Sessions [ENDORSED]
What is the difference between "Workshop", "Step-up", and "Drop-in" Sessions?
- Sat Jan 21, 2023 7:20 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Achieve HW 1 Question 9/10
- Replies: 4
- Views: 223
Re: Achieve HW 1 Question 9/10
Here is how you would do an ICE chart after adding [NO]
- Sat Jan 21, 2023 7:06 pm
- Forum: Chem 14B Uploaded Files (Worksheets, etc.)
- Topic: Lecture Slides
- Replies: 8
- Views: 387
Re: Lecture Slides
Open the Course Canvas page("lecture 1" or "lecture 2") and click "UCLA Media Reserves" to access lecture videos. You may also check the syllabus to see which chapters correspond with each week's lectures.
- Sat Jan 21, 2023 6:58 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook question topic 5G.3
- Replies: 2
- Views: 95
Textbook question topic 5G.3
The answer on the answer key is given in the form of pressure instead of concentration
(^{2}) instead of just
instead of just  ).
).
How do we know whether the expression for K should be in concentration or pressure.
(
How do we know whether the expression for K should be in concentration or pressure.

