Search found 106 matches
- Wed Mar 13, 2024 10:50 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Internal energy of an isothermal reaction
- Replies: 1
- Views: 59
Internal energy of an isothermal reaction
Why is the 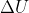 of an isothermal reaction equal to 0? I thought that isothermal would just mean that q=0, but this isn't the case in the entropy problems that we solved.
of an isothermal reaction equal to 0? I thought that isothermal would just mean that q=0, but this isn't the case in the entropy problems that we solved.
- Wed Mar 13, 2024 10:48 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy vs Enthalpy
- Replies: 2
- Views: 41
Re: Gibbs Free Energy vs Enthalpy
Gibbs free energy is a measure of the maximum work that a system can do. Enthalpy is a measure of the total heat of a system. Gibbs free energy is equal to enthalpy minus temperature x entropy.
- Wed Mar 13, 2024 10:46 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: K constant
- Replies: 2
- Views: 42
Re: K constant
Yes, because the relationship is Keq = k/k' you should be able to manipulate the equation given kreverse and Keq to find kfwd.
- Wed Mar 13, 2024 10:42 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow step
- Replies: 2
- Views: 30
Re: Slow step
I think this would usually be given to us in the problem, or it would be the step in the reaction mechanism that matches to the overall rate law from the experimental data.
- Wed Mar 13, 2024 10:40 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Textbook Problem 4.51
- Replies: 1
- Views: 35
Textbook Problem 4.51
This question asks why there is a discrepancy between the Gibbs free energy of vaporization of water calculated at 100C from Appendix A and the actual value. Why should the actual value be delta G = 0 at 100C? Why is the gibbs free energy zero for the normal boiling point?
- Wed Mar 06, 2024 5:31 pm
- Forum: General Rate Laws
- Topic: Unique Rate Law
- Replies: 3
- Views: 63
Re: Unique Rate Law
The unique rate law takes into the stoichiometric concentrations of the reactants and products so is the same for all reactants and products in the reaction.
- Wed Mar 06, 2024 5:28 pm
- Forum: First Order Reactions
- Topic: Textbook Problem 7B.3 part c
- Replies: 2
- Views: 36
Textbook Problem 7B.3 part c
Part C asks: 2A --> B + C given that [A] 0 = 0.153M and that after 115 s the concentration of B rises to 0.034M. Determine the rate constant for the first order reaction expressed for the rate of loss of A. I am confused about where to begin solving this problem since we are given an initial concent...
- Wed Mar 06, 2024 5:22 pm
- Forum: Zero Order Reactions
- Topic: Zero order units
- Replies: 3
- Views: 53
Zero order units
How would you derive the units of a rate constant for a zero-order reaction?
- Wed Mar 06, 2024 5:20 pm
- Forum: General Rate Laws
- Topic: Textbook Problem Section 7A Question 17A
- Replies: 1
- Views: 28
Re: Textbook Problem Section 7A Question 17A
When you are looking to find the order with respect to each species, you need to isolate where only that species has concentration changing. For B, you would use experiments 2 and 3 since only its concentrations are changing. When you divide the rate of experiment 3 by experiment 2, you get 5.84... ...
- Wed Feb 28, 2024 1:38 pm
- Forum: Balancing Redox Reactions
- Topic: Achieve question 2
- Replies: 1
- Views: 39
Re: Achieve question 2
To balance this reaction, you would need to add 2 H2O to the side with the BrO- so that the number of oxygens are equal. Then you would add 4OH- to that side and 4 H2O to the opposite side to balance the hydrogens. Finally, you balance charges by adding electrons. 2 of the waters should cancel out.
- Wed Feb 28, 2024 1:23 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Achieve #18
- Replies: 1
- Views: 45
Achieve #18
How would be balance an equation for the formation of Fe2O3 * 3H2O? I know that the reactants would be Fe, O2, and H2O.
I thought that it would be 4Fe + 3 O2 + 6 H2O --> 2 Fe2O3 * 6H2O, however this is the correct answer. Does anyone know how to go about doing this?
I thought that it would be 4Fe + 3 O2 + 6 H2O --> 2 Fe2O3 * 6H2O, however this is the correct answer. Does anyone know how to go about doing this?
- Wed Feb 28, 2024 12:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams Reactants and Products
- Replies: 1
- Views: 24
Cell Diagrams Reactants and Products
When writing cell diagrams, I thought that reactants were always written first and then products, however this isn't the case in all of the textbook problems. Could someone explain why this is and how to tell when you need to write products before reactants?
- Wed Feb 28, 2024 12:44 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Stoichiometric Coefficients and Voltage
- Replies: 2
- Views: 27
Re: Stoichiometric Coefficients and Voltage
Yes, this is because Ecell is an intensive property and it is measured per mole of electrons. So if you double the number of electrons in the equation, your Ecell would still be the same, because it is per mole of electrons (essentially you would be dividing the supposed larger E value by 2 so get t...
- Wed Feb 28, 2024 12:40 pm
- Forum: Balancing Redox Reactions
- Topic: Wk 7&8 Q#5
- Replies: 1
- Views: 40
Re: Wk 7&8 Q#5
One thing to remember is that in H2O2, oxygen has a -1 oxidation number. When balancing these half reactions, you are in basic solution, so you will be adding OH- to get the final balanced equations, not H+. You should get Cl2O7 + 6H20 + 8e --> 2 ClO2- + 3 H20 + 6OH- and H2O2 + 2 OH- --> O2 + 2 H2O ...
- Wed Feb 28, 2024 12:36 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration cell Ecell value
- Replies: 1
- Views: 20
Concentration cell Ecell value
Could someone re-explain why E cell (standard) = 0V for a concentration cell?
- Wed Feb 21, 2024 9:30 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cathode vs Anode
- Replies: 3
- Views: 50
Cathode vs Anode
Could someone explain how to determine which metal is the cathode and which is the anode?
- Wed Feb 21, 2024 9:29 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: orientations
- Replies: 1
- Views: 46
Re: orientations
In order to count the number of orientations, we would look at the different ways the atoms can be arranged in a molecule. For a simple example, nitric oxide could be written NO or ON. For more complex molecules, it is best to draw the Lewis structure and think about the different ways the molecule ...
- Wed Feb 21, 2024 9:25 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibb's Free Energy at Equilibrium
- Replies: 4
- Views: 71
Re: Gibb's Free Energy at Equilibrium
At equilibrium, the system has reached a rate where the forward and reverse reactions are occurring at equal rates. A system also tends towards minimizing free energy available to do work, and when it is at equilibrium, this energy is at a minimum (which is zero).
- Mon Feb 19, 2024 12:31 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy of Surroundings for Irreversible expansion
- Replies: 1
- Views: 48
Entropy of Surroundings for Irreversible expansion
Why is the entropy of surroundings equal to zero for isothermal irreversible expansion but not for reversible expansion since neither has a temperature change?
- Sun Feb 18, 2024 9:37 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy Definition
- Replies: 2
- Views: 31
Internal Energy Definition
What is a simple definition for internal energy and what it actually means, besides just the sum of heat and work?
- Sun Feb 18, 2024 9:26 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Ideal gas Assumptions
- Replies: 4
- Views: 96
Re: Ideal gas Assumptions
When we say that a gas is ideal, we assume that there are no intermolecular attractions or repulsions between the gas particles, that they are all equally sized and of negligible mass, and that their collisions are elastic. Also, the gas will expand to fill its entire container and the average kinet...
- Sun Feb 18, 2024 9:22 pm
- Forum: Phase Changes & Related Calculations
- Topic: Textbook Question 4.15
- Replies: 1
- Views: 38
Textbook Question 4.15
Hi, I am a bit stuck on how to start solving textbook problem 4.15. I wrote out my balanced equation of 2HCl (aq) + Zn (s) \rightarrow H 2 (g) + Zn 2+ (aq) + 2 Cl - (aq) the I know that the problem will involve the q=mc \Delta T equation, however I am not sure where to go from here. ...
- Wed Feb 14, 2024 8:47 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Constant pressure calorimeter
- Replies: 1
- Views: 28
Constant pressure calorimeter
For a calorimeter problem at constant pressure, how do we know that qtotal=0, i.e. that qsys=-qsurr?
- Tue Feb 13, 2024 4:55 pm
- Forum: Chem 14B Uploaded Files (Worksheets, etc.)
- Topic: Sam's Workshop Week 6
- Replies: 2
- Views: 177
Re: Sam's Workshop Week 6
Could you please post the answer key?
- Tue Feb 13, 2024 4:03 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Cp and Cv
- Replies: 2
- Views: 47
Re: Cp and Cv
Cp values will always be slightly higher than Cv values because under constant pressure the system is increasing temperature but also doing work of expansion (expanding its volume to keep the pressure the same) against an external pressure. Under a constant volume system, there is no work of expansi...
- Tue Feb 13, 2024 4:00 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Achieve #19
- Replies: 1
- Views: 19
Re: Achieve #19
equation should be  where the second delta G is delta G °
where the second delta G is delta G °
- Tue Feb 13, 2024 3:59 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Achieve #19
- Replies: 1
- Views: 19
Achieve #19
This question asks for delta G of the reaction at 298K when the partial pressures are P N2 = 0.3 bar, P H2 = 0.3 bar and P NH3 = 0.750 bar given that delta G ° = -32.8 kJ/mol for the reaction N2 (g) + 3 H2 (g) \rightleftharpoons 2 NH3 (g). To solve this problem, I was using the equation \Delta G = \...
- Mon Feb 12, 2024 11:42 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook 4B.5 - Dividing Rs (Gas Constants)
- Replies: 1
- Views: 24
Re: Textbook 4B.5 - Dividing Rs (Gas Constants)
Using these two different values for R depends on the units given in your problem/what units you are working with. If you are given pressure and volume values in atmospheres and Liters then it makes sense to use R = 0.08206 atmL/molK, but if you already have units in Joules then it makes sense to us...
- Mon Feb 12, 2024 11:40 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Textbook Question 4J.13
- Replies: 1
- Views: 34
Textbook Question 4J.13
This problem asks us to determine which of the compounds are stable with respect to decomposition into their elements under standard conditions. I wrote my first equation as PCl 5 (g) \rightarrow P(s) + 5/2 Cl 2 (g). However, the textbook has the equation reversed. I am confused why that would be th...
- Wed Feb 07, 2024 10:18 pm
- Forum: Phase Changes & Related Calculations
- Topic: Achieve 13 Week 3/4
- Replies: 2
- Views: 29
Re: Achieve 13 Week 3/4
A system doing work on the surroundings will have a negative value for w, because it is losing energy is push against the surroundings. Since w=-P\Delta V or w=-\Delta nRT , work will be negative when there is either an increase in the volume of the system or increase in the number of moles of gas. ...
- Wed Feb 07, 2024 10:14 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: How to determine when reaction is at constant pressure
- Replies: 2
- Views: 48
How to determine when reaction is at constant pressure
For textbook question 4D.7, we are asked to find the change in internal energy and must use the equation \Delta U=\Delta H-\Delta nRT , which, as we discussed in the example problem during lecture, applies to systems with a constant external pressure. However, this problem did not specifically state...
- Wed Feb 07, 2024 10:07 pm
- Forum: Phase Changes & Related Calculations
- Topic: Textbook 4C.7
- Replies: 1
- Views: 29
Re: Textbook 4C.7
For part a, you are given the heat required to vaporize 0.579 mol of methane. In order to find the enthalpy of vaporization of methane, which is a measurement of heat PER MOLE, you need to divide the 4.74 kJ by the 0.579 mol of methane. For part b, you will do the same thing, except first you must c...
- Wed Feb 07, 2024 10:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.9
- Replies: 1
- Views: 20
4E.9
For this question, I understand that we are supposed to calculate the different between having six resonance bonds between the carbons versus 3 C-C single bonds and 3 C=C double bonds. However, I don't understand why having a higher bond energy makes the resonance structure more stable and less reac...
- Mon Jan 29, 2024 8:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Finding Q given Kp
- Replies: 1
- Views: 18
Re: Finding Q given Kp
When calculating Kp (or Q) we will always use pressure in bars. However, since bars and atm have a roughly 1 to 1 conversion, we can use either of these units and come to the same answer.
- Mon Jan 29, 2024 8:09 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: NH4ClO4 Acidic Salt
- Replies: 2
- Views: 162
Re: NH4ClO4 Acidic Salt
ClO4- is the conjugate base of a strong acid, and therefore will have a negligible effect on the pH. NH4+ is the conjugate acid of a weak base (making it a stronger conjugate acid) and will therefore cause the solution to be acidic.
- Mon Jan 29, 2024 8:07 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Molar heat capacity Cp vs Cv
- Replies: 1
- Views: 38
Molar heat capacity Cp vs Cv
In lecture today we went over how constant pressure systems have a higher molar capacity than constant volume systems for gases but I didn't quite catch why. Could someone explain why this is the case?
- Mon Jan 29, 2024 8:03 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: ICE Table vs Henderson Hasselbach [ENDORSED]
- Replies: 1
- Views: 3657
ICE Table vs Henderson Hasselbach [ENDORSED]
How can we determine when to use an ICE table versus the henderson-hasselbach equation, specifically for buffer solutions?
- Tue Jan 23, 2024 10:01 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Achieve Weeks 2 and 3 questions 9 and 10
- Replies: 1
- Views: 44
Re: Achieve Weeks 2 and 3 questions 9 and 10
For an acidic solution, if the pH is larger than the pKa value, then the system will shift to create more H 3 O + to lower the pH and gain equilibrium. In that case, the system would be charged. For a basic solution, if the pH is larger than the pKa, the system will shift to reduce the OH - concentr...
- Tue Jan 23, 2024 9:58 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook Question 4B.9
- Replies: 2
- Views: 104
Textbook Question 4B.9
This question refers to an adiabatic process where no energy is transferred as heat in a closed system. I am confused why it is always true that w=0 and always false that w < 0. Even though no heat is being transferred, closed systems allow for the exchange of energy with the surroundings. Why can't...
- Mon Jan 22, 2024 8:31 pm
- Forum: Calculating Work of Expansion
- Topic: Homework 4A.3
- Replies: 1
- Views: 81
Re: Homework 4A.3
For this problem, it is helpful to recognize that \Delta V=-Axd . In this case, Area would be equal to \pi r 2 , where r is half the diameter. This is because we have a tube which would have a cross section as a circle. The value of d would be 20 cm. From there you should be able to calculate the vo...
- Mon Jan 22, 2024 8:27 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Approximation using Ka vs using Kc for equilibrium problems
- Replies: 1
- Views: 39
Approximation using Ka vs using Kc for equilibrium problems
During the chemical equilibrium unit, we used an approximation when Kc < 1x10-4 yet when doing acid-base problems we learned today that we can approximate generally when Ka < 1x10-3. Why is there this difference? And what does it have to do with the 5% rule?
- Tue Jan 16, 2024 10:31 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Achieve #3 calculating pH
- Replies: 1
- Views: 36
Achieve #3 calculating pH
Question 3 on Achieve asks to find the percent ionization, pH and pOH for lactic acid given a 0.1125 M solution and Ka = 8.40x10 -4 . I correctly calculated the percent ionization to be 8.28%, however I am confused as to why we can calculate the pH using the x-value of H 3 O + concentration. I thoug...
- Mon Jan 15, 2024 7:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pH of polyprotic acids
- Replies: 1
- Views: 48
pH of polyprotic acids
I am confused when to use this equation provided in the textbook: pH = 1/2 (pKa1 + pKa2) versus when to just assume that the second de-protonation is negligible to the pH value and only use Ka1. Does anyone know when specifically to use that equation?
- Mon Jan 15, 2024 7:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6E.1A textbook problem
- Replies: 1
- Views: 67
Re: 6E.1A textbook problem
Hi, so for this problem H2SO4 is going to first de-protonate in water into H3O+ and HSO4-. This would be a complete deprotonation, so the H3O+ concentration would equal the original concentration of H2SO4, which is 0.05 M. Next, you would use the equation for the second deprotonation (which is a par...
- Mon Jan 15, 2024 12:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Composite Equations
- Replies: 1
- Views: 48
Re: Composite Equations
When you are adding two equations together, it is helpful to see if there are any intermediate substances that will be consumed during the overall reaction. For instance, if your two equations are [A] + [B] \leftrightharpoons [C] and [C] + [D] \rightleftharpoons [E] then your overall equation would ...
- Wed Jan 10, 2024 1:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Approximating
- Replies: 2
- Views: 33
Re: Approximating
We can only approximate that the initial and final molar concentrations for the reactants will be the same when Kc is less than or equal to 10 -4 . When Kc is larger than this, the change in x will be significant enough that the molar concentration of the reactants at equilibrium will vary from the ...
- Tue Jan 09, 2024 10:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Activity, aJ
- Replies: 1
- Views: 46
Re: Activity, aJ
Hi! In the audio-visual focus topics Dr. Lavelle said that in this class we will assume the activity coefficient is equal to one and consider activity to be equal to concentration. The activity for solid and liquid is 1 because the molar concentration of a pure substance (liquid or solid) does not c...
- Mon Jan 08, 2024 9:04 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Reaction shifting vs equilibrium shifting
- Replies: 2
- Views: 59
Reaction shifting vs equilibrium shifting
I understand that the equilibrium constant is temperature dependent, so a change in temperature will cause the equilibrium constant (K) to change. Does this still mean that when the temperature of a reaction occurs it will either shift to form more products or reactants or is only K changing? Simila...
- Mon Jan 08, 2024 1:38 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc vs K vs Kp
- Replies: 1
- Views: 38
Kc vs K vs Kp
I know the difference between Kc and Kp is that Kc deals with equilibrium concentrations and Kp deals with partial pressures, but the book also differentiates between just K and Kc. What would be the difference here? Also can gases only be represented using Kp or can they also be expressed using Kc?
- Thu Dec 07, 2023 9:09 pm
- Forum: Lewis Acids & Bases
- Topic: Textbook Question 6.21
- Replies: 1
- Views: 84
Textbook Question 6.21
This question gave the thymine structure and asked how many protons the base can accept. I am confused why the oxygens with 2 lone pairs cannot accept a proton. The answer is that only the two nitrogens can accept a proton.
- Tue Dec 05, 2023 11:03 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Boiling Point Temps N2, NO2, NO
- Replies: 2
- Views: 527
Boiling Point Temps N2, NO2, NO
What would be the order of increasing boiling point temperatures for N2, NO2 and NO? I understand that N2 would have the lowest boiling point because it only experiences LDFs, however I am not sure how to differentiate NO2 and NO since both experience dipole-dipole interactions.
- Tue Dec 05, 2023 11:01 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Boiling Point Temps N2, O2, NO
- Replies: 2
- Views: 1628
Boiling Point Temps N2, O2, NO
What would be the order of increasing boiling point temperatures for N 2 , O 2 and NO? I understand that NO would have the highest boiling point because it experiences dipole-dipole forces. To determine the difference between N2 and O2 we would look at size since the larger molecule would be more po...
- Mon Dec 04, 2023 9:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Diethylenetriamine charge
- Replies: 1
- Views: 63
Diethylenetriamine charge
I know ethylenediamine has no charge and EDTA has a charge of 4-, but what is the charge on diethylenetriamine?
- Mon Dec 04, 2023 9:39 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Boiling Point and IMFs
- Replies: 2
- Views: 317
Boiling Point and IMFs
How would you determine the order of boiling temperatures for N2, O2 and NO since all of these compounds are linear and only experience LDFs?
- Mon Dec 04, 2023 9:37 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Porphyrin Ligand
- Replies: 2
- Views: 87
Porphyrin Ligand
Would the porphyrin ligand be considered chelating since there are 3 spaces in between the two lone pairs on the nitrogen? In class we discussed having a lone pair-spacer-spacer-lone pair to be a chelating ligand. Does that mean that there is too great an angle for this to occur in porphyrin?
- Tue Nov 28, 2023 9:22 pm
- Forum: Hybridization
- Topic: 2F.15
- Replies: 1
- Views: 43
Re: 2F.15
You would expect the bond angle to increase because as you gain s character you are moving more towards an sp2 hybridization rather than an sp3 hybridization and the sp2 has a higher bond angle.
- Tue Nov 28, 2023 9:19 pm
- Forum: Bronsted Acids & Bases
- Topic: Textbook Question 6.21
- Replies: 1
- Views: 45
Textbook Question 6.21
Given the thymine structure, we were asked to determine how many protons this base could accept. I understood that each nitrogen could accept one H+ ion, however I am not sure why the two oxygen atoms in the structure cannot accept protons since they have electron pairs available for bonding.
- Tue Nov 28, 2023 2:58 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: HF vs H2O Strength
- Replies: 2
- Views: 590
HF vs H2O Strength
I have seen explained that HF is a stronger acid than H2O because F is more electronegative than O. However, I am confused about why we can use electronegativities for comparison because when looking at HF vs HCl, F is more electronegative than Cl but HCl is considered the stronger acid since it can...
- Sun Nov 26, 2023 10:49 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: HF vs HCl strength
- Replies: 2
- Views: 137
HF vs HCl strength
Why would HCl be a stronger acid than HF? I thought that since F is more electronegative than Cl this would make the bond more polar and therefore make HF a stronger acid. Why is it that bond strength takes over in this case?
- Sun Nov 26, 2023 8:27 pm
- Forum: Naming
- Topic: Achieve question 1
- Replies: 3
- Views: 84
Achieve question 1
This question asked for the systematic name for [Co(NH3)5Cl]Cl2.
Using the rules in the textbook, I got pentaammine chlorido cobalt (III) chloride however this is not correct. I was wondering if anyone could tell me what part of this naming needs to be corrected.
Using the rules in the textbook, I got pentaammine chlorido cobalt (III) chloride however this is not correct. I was wondering if anyone could tell me what part of this naming needs to be corrected.
- Wed Nov 22, 2023 10:25 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Textbook Problem 9C5
- Replies: 1
- Views: 30
Textbook Problem 9C5
Why can CO32- be both bidentate and monodentate?
- Mon Nov 20, 2023 10:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR for molecules with multiple central atoms
- Replies: 2
- Views: 39
Re: VSEPR for molecules with multiple central atoms
Larger, more complex compounds may have multiple central atoms, in which case you would determine the VSEPR notation around each atom.
- Mon Nov 20, 2023 10:38 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pairs and Bond Angles
- Replies: 2
- Views: 40
Re: Lone Pairs and Bond Angles
Lone pairs take up more volume than bonding atoms, so they will repel these other bonded atoms and thus change the bond angles to take up more space.
- Mon Nov 20, 2023 10:11 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: NO2- dentate
- Replies: 1
- Views: 204
NO2- dentate
Why is NO2- ambidentate and not bidentate even though the nitrogen and oxygens have lone pairs?
- Wed Nov 15, 2023 8:00 pm
- Forum: Bond Lengths & Energies
- Topic: Covalent and Ionic Bond Lengths
- Replies: 2
- Views: 61
Re: Covalent and Ionic Bond Lengths
First you need to draw the Lewis structure of the molecule to determine the number of bonds between the two atoms. Then you use the atomic radii of the two atoms for that specific number of bonds and add them together to get the bond length.
- Wed Nov 15, 2023 7:57 pm
- Forum: Lewis Acids & Bases
- Topic: lewis Acids and Bases
- Replies: 2
- Views: 74
Re: lewis Acids and Bases
Water can accept and become H3O+ or lose and become OH-.
- Wed Nov 15, 2023 7:55 pm
- Forum: Octet Exceptions
- Topic: Halogen bonding
- Replies: 1
- Views: 106
Halogen bonding
Why do the halogens only like to form single bonds and typically won't form double or triple bonds?
- Wed Nov 15, 2023 7:53 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipole forces and nonpolar molecules
- Replies: 2
- Views: 95
Dipole forces and nonpolar molecules
Can a nonpolar molecule have dipole-dipole forces if it has polar bonds?
- Sat Nov 11, 2023 3:00 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Ion-dipole vs H-bond
- Replies: 1
- Views: 59
Ion-dipole vs H-bond
One of the review sheets has ion-dipole interactions stronger than H-bonds and the other has the opposite. Which of these two interactions are stronger and why?
- Tue Nov 07, 2023 8:26 pm
- Forum: Bond Lengths & Energies
- Topic: Achieve hw #11
- Replies: 2
- Views: 46
Re: Achieve hw #11
This is because hydrogen has a smaller atomic radius than oxygen. Hydrogen doesn't follow the periodic trend from left to right across the table if that is what confused you.
- Tue Nov 07, 2023 8:21 pm
- Forum: Ionic & Covalent Bonds
- Topic: Syllabus Problem 2D 19
- Replies: 2
- Views: 26
Re: Syllabus Problem 2D 19
Figure 2D.11 gives you the covalent radii of each atom in these structures. For each of these, you have the central atom (C, Si, or Sn) bonded to 4 fluorine atoms. Therefore, you know that your bonds are going to be C--F, Si--F, and Sn--F. To find the bond length, you simply add the radii of the cen...
- Tue Nov 07, 2023 8:14 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: # of valence electrons in Fe
- Replies: 5
- Views: 306
Re: # of valence electrons in Fe
I believe iron would have two valence electrons. If we look at its electron configuration, it is [Ar] 3d 6 4s 2 . The two electrons in the 4s orbital would be the valence electrons since this is the highest energy shell and valence electrons come from the s and p orbitals. The presence of the 3d orb...
- Tue Nov 07, 2023 8:04 pm
- Forum: Electronegativity
- Topic: Achieve #9
- Replies: 2
- Views: 106
Achieve #9
Can someone explain why oxygen is more electronegative than chlorine in the perchlorate ion? I am also still confused on how to determine which Lewis structure is most plausible based on the oxidation number. I understand that chlorine has an oxidation number of +7, but I'm not sure where to go from...
- Sun Nov 05, 2023 5:27 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Textbook Question 3F.19
- Replies: 1
- Views: 69
Textbook Question 3F.19
This question asked to explain why the vapor pressure of diethyl ether is greater than that of water in terms of the strength and type of intermolecular forces present. To my knowledge, water is able to hydrogen bond with other molecules while diethyl ether is only able to have London interactions a...
- Wed Nov 01, 2023 8:43 pm
- Forum: Resonance Structures
- Topic: Textbook Question 2C.3
- Replies: 1
- Views: 41
Textbook Question 2C.3
This question asked to draw the Lewis structure and typical contributions to resonance for chloric acid HClO 3 . I was curious why there would be multiple structures for this compound since there is one structure where all of the formal charges on the atoms are zero but then on the other ones some o...
- Wed Nov 01, 2023 8:36 pm
- Forum: Lewis Structures
- Topic: NO3- Structure
- Replies: 3
- Views: 54
Re: NO3- Structure
This is because NO 3 - has resonance. Drawing a single lewis structure, the nitrogen will form a double bond with one of the oxygens because this will reduce the formal charge and make the structure more favorable. As we learned, these electrons are really delocalized around all the atoms, the syste...
- Wed Nov 01, 2023 8:31 pm
- Forum: Lewis Structures
- Topic: Textbook Question 2B.15
- Replies: 1
- Views: 38
Textbook Question 2B.15
This question asks to draw the Lewis structures that contribute to resonance hybrid of nitryl chloride, ClNO 2 . For this drawing, I had the nitrogen as the central atom since it has the lowest ionization energy, then I had the oxygens across from each other and the chlorine coming off the nitrogen....
- Wed Nov 01, 2023 8:23 pm
- Forum: Lewis Structures
- Topic: Textbook Question 2B.11
- Replies: 1
- Views: 28
Textbook Question 2B.11
Why does the Lewis structure for glycine, H2C(NH2)COOH have the double bond attached to the free oxygen and not the oxygen attached to the hydrogen atom?
- Mon Oct 30, 2023 11:06 pm
- Forum: Octet Exceptions
- Topic: sulfate ion expanded octet
- Replies: 1
- Views: 39
sulfate ion expanded octet
Today in class we drew the Lewis structure for the sulfate ion and determined that sulfur can have more than an octet because the 3d states can be involved in bonding. I was a bit confused about this since sulfur only goes up to 3p 4 in terms of its electron configuration and does not have any 3d or...
- Tue Oct 24, 2023 8:39 pm
- Forum: Hybridization
- Topic: Valence electrons
- Replies: 2
- Views: 105
Re: Valence electrons
Valence electrons are simply the ones in the outermost shell (principal quantum number). So in an electron configuration that has 3d6 4s2 for instance, the valence electrons would be in the 4s orbital.
- Tue Oct 24, 2023 8:35 pm
- Forum: Properties of Electrons
- Topic: Velocity from wavelength and frequency
- Replies: 2
- Views: 79
Re: Velocity from wavelength and frequency
Hi, I think the equation you are using is wavelength times frequency equals the speed of light, but this doesn't apply to any particle (or electron), just light. If you are trying to find the velocity of an electron, you should use the de Broglie equation which is wavelength = h/mv. Rearranging this...
- Tue Oct 24, 2023 8:32 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: half filled subshell stability
- Replies: 1
- Views: 33
Re: half filled subshell stability
Half filled subshells are more stable because of the symmetric distribution of electrons in each orbital. If you think of the electron configuration diagram, each orbital has one unpaired electron with the same (parallel) spin. This state reduces repulsions and therefore increases stability.
- Sun Oct 22, 2023 11:50 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Achieve week 2,3,4 question #12
- Replies: 1
- Views: 74
Re: Achieve week 2,3,4 question #12
In this problem, it states that electron affinity is equal to the difference in energy of the incident photon (the incoming light) and the ejected electron. Here, the equation would be similar to the one we used in class with the work function: E photon -E e = E affinity . Therefore, E affinity =E p...
- Sun Oct 22, 2023 11:39 pm
- Forum: DeBroglie Equation
- Topic: Achieve Question 17
- Replies: 2
- Views: 68
Re: Achieve Question 17
Hi, since this is a diatomic atom, you would double the atomic mass. Then you would need to convert to kg/mol (dividing grams by 1000 to get to kilograms). However, this is for 1 mol of chlorine molecules and we are being asked to find the wavelength for a single molecule. Since the conversion is 6....
- Thu Oct 19, 2023 9:44 pm
- Forum: Limiting Reactant Calculations
- Topic: Fundaments M13
- Replies: 1
- Views: 348
Fundaments M13
I am unsure how to approach this problem and was hoping someone could help me out. I converted each product amount to moles of C and H respectively but am not sure if that is necessary or what to do from there. PCBs were once widely used industrial chemicals but were found to pose a risk to health a...
- Wed Oct 18, 2023 9:17 pm
- Forum: DeBroglie Equation
- Topic: de Broglie and light equations
- Replies: 1
- Views: 76
de Broglie and light equations
In class, Dr. Lavelle emphasized that the equations E=hv and E=pc can only be applied to light, yet they are used to derive the de Broglie equation \lambda =\frac{h}{p} , which cannot be applied to light, only objects with rest mass. Can someone explain this to me? How can equations that only apply ...
- Tue Oct 17, 2023 11:25 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Radial distribution function
- Replies: 1
- Views: 111
Radial distribution function
In the textbook, the section on orbital shapes in topic 1D covered the radial distribution function. I was wondering if someone could explain what this means and how to apply it because I didn't really understand the book's definition.
- Tue Oct 17, 2023 10:50 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: HW Q 11
- Replies: 2
- Views: 39
Re: HW Q 11
When you are using the Rydberg equation, recall that it is v = R[1/n 1 2 - 1/n 2 2 ]. This equation uses the frequency of the light, however what you have set up uses the wavelength. Therefore, you first need to convert 434 nm to its corresponding wavelength using the speed of light equation c= \lam...
- Tue Oct 17, 2023 1:22 pm
- Forum: Properties of Light
- Topic: Focus 1A Question 9
- Replies: 2
- Views: 55
Re: Focus 1A Question 9
For me, it helped to look at the one diagram from class which had the electromagnetic spectrum with each type of light and its corresponding wavelength. Since making popcorn had the longest wavelength, this would be found in the microwave region, which has the longest wavelengths on that graph. Sunt...
- Tue Oct 17, 2023 1:15 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Textbook Question 1.D.13
- Replies: 2
- Views: 53
Re: Textbook Question 1.D.13
I believe you are actually looking at question 14 in the textbook. Regardless, for part a, when n=6, l could equal 0, 1, 2, 3, 4, or 5 so there are therefore 6 possible values for l. Remember that the value of l essentially gives you the shape of the orbital, where 0 is s, 1 is p, and so on. For par...
- Sun Oct 15, 2023 11:45 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Boundary Surface
- Replies: 1
- Views: 79
Re: Boundary Surface
The boundary surface is a smooth surface enclosing the electron cloud and it is used to draw orbitals, indicating the probability of where the electron could be located. I believe the boundary surface is something more conceptual than used for calculations, but someone else may be able to answer the...
- Sun Oct 15, 2023 11:40 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Achieve Week 2 Question 8
- Replies: 3
- Views: 52
Re: Achieve Week 2 Question 8
Yes, you are on the right track! From there you need to find the change in energy (E final -E initial -- i.e. E 1 -E 7 ) when going from energy level n=7 to n=1. Then you can combine the equations E=hv and c = \lambda v to form E=\frac{hc}{\lambda } . Rearrange for wavelength, plug in your energy va...
- Sun Oct 15, 2023 11:00 pm
- Forum: Properties of Electrons
- Topic: Achieve Question 7
- Replies: 1
- Views: 58
Achieve Question 7
As you may know, placing metal objects into a microwave can generate sparks. Two of your friends are arguing over the cause of sparks. One says that microwaves "herd" electrons into "pointy" areas of the metal object from which electrons jump from one part of object to another. O...
- Thu Oct 12, 2023 11:55 pm
- Forum: Photoelectric Effect
- Topic: Atomic vs Molecular Spectroscopy
- Replies: 1
- Views: 35
Atomic vs Molecular Spectroscopy
Hi, could someone explain the difference between atomic and molecular spectroscopy? My understanding so far is that atomic spectroscopy deals with the EMR emitted/absorbed by atoms while molecular spectroscopy deals with molecules. Are there any other distinctions or differences in the graphs create...
- Thu Oct 12, 2023 4:24 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: E = -hR/n^2 interpretation
- Replies: 1
- Views: 43
E = -hR/n^2 interpretation
Today in discussion we talked about how the equation E = \frac{-hR}{n^{2}} says that E ∝ - \frac{1}{n^{2}} . I have written in my notes that this relationship tells us that as you increase the energy level (say from n=1 to n=2) then the energy also increases. I am a bit confused about how this conce...
- Wed Oct 11, 2023 8:25 pm
- Forum: Properties of Light
- Topic: Absorption vs Emissions Spectra
- Replies: 2
- Views: 63
Absorption vs Emissions Spectra
Today in class we went over that either absorption or emissions spectroscopy can be used to calculate the change in energy for an electron to go from one energy level to another, and thus also the frequency of light needed to energize an electron from one principle quantum level to another. However,...
- Wed Oct 11, 2023 8:21 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Energy of bound and free Electrons
- Replies: 2
- Views: 54
Re: Energy of bound and free Electrons
Basically, as an electron gets farther and farther from the nucleus (going to higher energy levels), the nucleus has a weaker hold on that electron (its effective nuclear charge is reduced for that electron). Eventually, you get to a point when the electron is so far away that there is no attraction...
- Wed Oct 11, 2023 8:17 pm
- Forum: Properties of Light
- Topic: Weeks 2,3,4 Assignment Question 2
- Replies: 3
- Views: 61
Re: Weeks 2,3,4 Assignment Question 2
If you recall from the equation c= \lambda v, the speed of light = wavelength x frequency. What this tells us is that frequency and wavelength are inversely proportional (as one increases, the other decreases) but that the speed of the electromagnetic radiation (EMR) is constant at 3.0x10 8 m/s. A h...
- Wed Oct 11, 2023 8:09 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Calculating number of Photons
- Replies: 1
- Views: 52
Re: Calculating number of Photons
In order to find the number of photons, you need to divide the total energy (in Joules) for the 32W lamp (think of this as every single photon) by the energy for an individual photon (which you said that you calculated in joules). The units of joules will cancel out and you should be left with the n...
- Mon Oct 09, 2023 4:08 pm
- Forum: Photoelectric Effect
- Topic: EK = 1/2mv^2
- Replies: 3
- Views: 71
Re: EK = 1/2mv^2
The E K = 1/2 mv 2 equation gives the velocity of the resultant ejected electron and is simply incorporated into the equation E photon - E threshold (work function) = E k . What this equation basically says is that the ejected electron will have energy equal to the energy of the light of the incomin...

