Search found 70 matches
- Sat Mar 14, 2015 11:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: How to determine which chemicals produce a high voltage
- Replies: 7
- Views: 10966
Re: How to determine which chemicals produce a high voltage
There was a typo in my original post. It read: - reduction of Fe2+ to Fe3+ (0.77 V) It should have said (and it's updated): - reduction of Fe3+ to Fe2+ (0.77 V) To answer your other questions: The zinc will be oxidized in the anode half-cell and the iron (iii) will be reduced in the cathode half-cel...
- Sat Mar 14, 2015 3:33 pm
- Forum: General Rate Laws
- Topic: First order and second order reaction rates
- Replies: 3
- Views: 2888
Re: First order and second order reaction rates
When you're looking at a rate law, a first order reaction will have an overall order of one (the sum of all the exponents will be 1) and a second order reaction will have an overall order of 2 (the sum of all the exponents will be 2). The exponents can be determined experimentally (for reactions wit...
- Sat Mar 14, 2015 1:52 am
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: 2010 final Q7B naming cis/trans
- Replies: 2
- Views: 714
Re: 2010 final Q7B
Anytime there is a possibility of the molecule having an isomer, you have to indicate if it's cis- or trans-. Notice that there are two ways you can draw the molecule if cis- or trans- isn't specified (you could draw the fourth carbon going up from the third carbon and it would have the same molecul...
- Sat Mar 14, 2015 1:35 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: How to determine which chemicals produce a high voltage
- Replies: 7
- Views: 10966
Re: How to determine which chemicals produce a high voltage
You are essentially correct. You basically just look at all the half reactions you can form with the given chemicals and compare them to find the highest combination. You would get the highest combination by taking the half reaction with the largest negative value and the half reaction with the larg...
- Fri Mar 13, 2015 6:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 2010 Final #2A RXN Enthalpy
- Replies: 2
- Views: 635
Re: 2010 Final #2A RXN Enthalpy
I don't think you labeled the problem correctly. I found this in my course reader in the 2010 Midterm, Q2B (you may want to edit your title). To answer your question: you're right that you have to flip the original equation that you have, but notice the coefficients are different. The original equat...
- Fri Mar 13, 2015 1:17 am
- Forum: *Alkanes
- Topic: final 2012 Q6D: (Z) or (E) forms of a pentene
- Replies: 3
- Views: 711
Re: final 2012 Q6D: (Z) or (E) forms of a pentene
Here's a picture showing you both the E/Z forms of the molecule. I just took the molecule from the answer in the practice final and rotated it on its side to focus on the double bond. The stars indicate the priority molecule on either side of the double bond.
- Thu Mar 12, 2015 11:04 pm
- Forum: *Alkanes
- Topic: Naming of Hydrocarbons on the Final
- Replies: 2
- Views: 806
Re: Naming of Hydrocarbons on the Final
I'm pretty sure Professor Lavelle said that both the common names and IUPAC naming are acceptable. So knowing either one should work.
- Thu Mar 12, 2015 5:11 pm
- Forum: First Order Reactions
- Topic: KemiStry with Kayla and Sarah - Quiz 2 Review!
- Replies: 6
- Views: 2799
Re: KemiStry with Kayla and Sarah - Quiz 2 Review!
Integration is just reversing the process of finding the derivative, so you would add one to the exponent and divide by the new exponent (whereas in deriving something, you multiple by the exponent and then subtract one from the exponent).
- Thu Mar 12, 2015 4:56 pm
- Forum: First Order Reactions
- Topic: KemiStry with Kayla and Sarah - Quiz 2 Review!
- Replies: 6
- Views: 2799
Re: KemiStry with Kayla and Sarah - Quiz 2 Review!
To go from [A]-2 to -[A]-1, you have to integrate it, not derive it.
But you're right, if we were to derive [A]-2, we would get -2[A]-3.
But you're right, if we were to derive [A]-2, we would get -2[A]-3.
- Wed Mar 11, 2015 4:43 pm
- Forum: *Alkanes
- Topic: Geometric isomers for C6H14!
- Replies: 4
- Views: 1484
Re: Geometric isomers for C6H14!
I'm not sure about your first question. For your second question, you can write a molecular formula from a line structure by going from left to right and writing down what you see. For example, for 2-methylpentane, the molecule starts with a CH3 on the leftmost side, followed by two CH2's (which the...
- Wed Mar 11, 2015 4:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Finding the n value for a redox reaction!
- Replies: 2
- Views: 3937
Re: Finding the n value for a redox reaction!
The overall redox reaction given in the problem is 2Fe(s) + 2H_{2}O(l) + O_{2}(aq) \rightarrow 2Fe(OH)_{2}(s) . The way that I was able to determine which half reaction to use was by looking at the products and reactants given and the overall standard cell pot...
- Mon Mar 09, 2015 9:20 pm
- Forum: *Cycloalkenes
- Topic: Diene
- Replies: 4
- Views: 1139
Re: Diene
Yes, we would use the same Greek prefixes used to label multiple substituents. So three double bonds would be indicated by "tri-en-".
- Wed Mar 04, 2015 12:18 am
- Forum: Zero Order Reactions
- Topic: How can a reaction be zero order?
- Replies: 2
- Views: 2946
Re: How can a reaction be zero order?
I'm not sure if it means that the reaction depends only on the activation energy or the catalyst, but the way I picture zero order reactions is like this: Picture a hemoglobin molecule (this is going to be a very simplified picture of the body). Each hemoglobin molecule can only carry four oxygen mo...
- Tue Mar 03, 2015 11:40 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated System Energy Change
- Replies: 2
- Views: 672
Re: Isolated System Energy Change
By definition, energy and matter cannot be transferred into/out of an isolated system, so whatever energy is in the system remains constant. Therefore, if an isolated system has +15 kJ of energy at some point in time, it will have +15kJ at any other time. Yes, the amount of energy would remain const...
- Mon Mar 02, 2015 6:55 pm
- Forum: *Nucleophiles
- Topic: Is benzene a common name for cyclohexene?
- Replies: 2
- Views: 2133
Re: Is benzene a common name for cyclohexene?
They have difference numbers of hydrogen atoms attached to the carbons and a different number of double bonds and that's probably the biggest difference, benzene is a completely different class. The number of carbons is just one part the nomenclature, you also have to consider what else makes up the...
- Wed Feb 25, 2015 11:52 pm
- Forum: First Order Reactions
- Topic: If slope for ln(A) vs. t is negative is k?
- Replies: 7
- Views: 11087
Re: If slope for ln(A) vs. t is negative is k?
Martha, I don't think rates can be negative either. If k is always positive and concentrations are always positive, the rate is always positive as well. Correct me if I'm wrong, but even if the rate is for decomposition / consumption and has a negative sign, the rate is still actually positive, beca...
- Wed Feb 25, 2015 10:30 pm
- Forum: First Order Reactions
- Topic: Stoichiometric Coefficient impact?
- Replies: 1
- Views: 766
Re: Stoichiometric Coefficient impact?
No, I believe the stoichiometric coefficients wouldn't change anything in regards to the half-life. For first order reactions, the half-life is only dependent on k (t = ln2 / k). So if we're given the time, that's all we need to solve the problem. To go from 1 whole quantity to half that quantity ta...
- Wed Feb 25, 2015 12:25 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Reactants that don't play a part in the reaction
- Replies: 2
- Views: 927
Re: Reactants that don't play a part in the reaction
I think we exclude the Ar because it occurs as a reactant and a product, so it would just cancel out. Molecularity is determined by the number of reactants that take part in an elementary reaction, so even though the Ar is canceled out, it is still used up in the elementary step, so it contributes t...
- Tue Feb 24, 2015 9:27 pm
- Forum: First Order Reactions
- Topic: If slope for ln(A) vs. t is negative is k?
- Replies: 1
- Views: 750
Re: If slope for ln(A) vs. t is negative is k?
The negative sign is added so that the slope can be negative while k remains positive, so if the slope was -3:
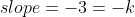
and k is just 3 (still positive).
Just remember, the proportionality constant, k, is always positive.
and k is just 3 (still positive).
Just remember, the proportionality constant, k, is always positive.
- Tue Feb 24, 2015 2:59 am
- Forum: General Rate Laws
- Topic: Negative Rate Constant?
- Replies: 4
- Views: 22877
Re: Negative Rate Constant?
Also, in first/zero order reactions, as Tatyana mentioned, the straight line plot has slope -k, but we have to add the negative sign because k has to remain positive, but the line has a downward slope.
- Tue Feb 24, 2015 12:42 am
- Forum: General Rate Laws
- Topic: Negative Rate Constant?
- Replies: 4
- Views: 22877
Re: Negative Rate Constant?
Another way to think of it is that reactions rates are always positive and since k is a proportionality constant that relates some given concentration(s) (which are always positive) with the rate (also always positive), it's not possible for k to be negative.
- Sun Feb 22, 2015 10:24 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation
- Replies: 1
- Views: 696
Re: Arrhenius Equation
The Arrhenius Equation doesn't depend on the order of the reaction. It just shows the temperature dependence of k, so yes, the Arrhenius Equation does apply to reactions of any order.
- Sun Feb 22, 2015 7:21 pm
- Forum: Second Order Reactions
- Topic: 14.33 Equation for the half-life of a 2nd order reaction
- Replies: 2
- Views: 4644
Re: 14.33 Equation for the half-life of a 2nd order reaction
On Prof. Lavelle's Constants and Equations, the last line gives the two relevant equations for this problem. For second order reactions, use \frac{1}{[A]} = kt + \frac{1}{[A]}_{0} and t_{1/2} = \frac{1}{k[A]_{0}} (the sheet uses R instead of A, but I used A because that's what the solutions manual u...
- Sun Feb 22, 2015 6:59 pm
- Forum: First Order Reactions
- Topic: When given t_1/2
- Replies: 1
- Views: 687
Re: When given t_1/2
Since the logic for your method is the same as the logic used in parts a and b, I believe you could use your method to solve a problem like that. But I believe you mixed up the equation in your description. (\frac{1}{2})^{n} = \frac{[A]}{[A]_{0}} (so the ratio of final concentration to initi...
- Sun Feb 22, 2015 4:01 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: (Hw prob 14. 23) Finding rate constant for 1st order rxn
- Replies: 2
- Views: 720
Re: (Hw prob 14. 23) Finding rate constant for 1st order rxn
That's just the initial concentration of A subtracted by the change in A.
The change in A is stoichiometrically determined using the change in B (A decreases twice as fast as B increased).
The change in A is stoichiometrically determined using the change in B (A decreases twice as fast as B increased).
- Sat Feb 21, 2015 2:23 pm
- Forum: General Rate Laws
- Topic: Unique rate law
- Replies: 5
- Views: 2285
Re: Unique rate law
Yeah, I didn't think the "unique rate law" was a thing, so I was just checking.
- Fri Feb 20, 2015 9:10 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Substituting time for k
- Replies: 2
- Views: 7832
Re: Substituting time for k
The units for the rate of milk spoiling are \frac{[milk]}{hr} . I believe we assume a first order reaction, so rate = k[milk] . Then, the units of k and k' are both \frac{1}{hr} . This is why you can rewrite \frac{k}{k'} as \frac{t'}{t} since k and t (time in hours) are inversely related. We...
- Fri Feb 20, 2015 8:48 pm
- Forum: General Rate Laws
- Topic: Unique rate law
- Replies: 5
- Views: 2285
Re: Unique rate law
You're referring to the unique average rate (not unique rate law), right? Other than that, everything else you said seems correct.
- Fri Feb 20, 2015 2:15 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Missing - sign on 14.45?
- Replies: 1
- Views: 641
Re: Missing - sign on 14.45?
I'm assuming you're talking about the Cl at the end of the first step, and yes, it should have a negative sign since it's the intermediate chloride ion that the solutions manual references.
- Thu Feb 19, 2015 11:08 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Determining initial rate of decamp of N2O5
- Replies: 1
- Views: 644
Re: Determining initial rate of decamp of N2O5
The value plugged into the rate law is the concentration of 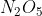 and we get that by converting the grams to moles and dividing by the total volume. The grams given already tells us the quantity of
and we get that by converting the grams to moles and dividing by the total volume. The grams given already tells us the quantity of 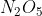 in the reaction, so we don't need to multiply it by two.
in the reaction, so we don't need to multiply it by two.
- Wed Feb 18, 2015 9:48 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation for two temperatures (14.59)
- Replies: 3
- Views: 1354
Re: Arrhenius Equation for two temperatures (14.59)
The derivation for that equation can be found on page 589 of the textbook. Essentially, you're just subtracting two Arrhenius equations for different T and k values (in the same way we were able to subtract one Van't Hoff equation from another). T and k correspond to one pair of values and T' and k'...
- Wed Feb 18, 2015 1:04 am
- Forum: General Rate Laws
- Topic: Bimolecular rate law, 14.47b and 14.49
- Replies: 3
- Views: 1130
Re: Bimolecular rate law, 14.47b and 14.49
Problem 14.47 refers you to Table 14.3 (on page 581) and that might help you understand how to write the rate law for an elementary step. For elementary reactions, we use the coefficients to write the rate law for the step. So in the case of 14.47b, the solutions manual is correct. Since each of the...
- Wed Feb 18, 2015 12:49 am
- Forum: General Rate Laws
- Topic: Overall Order
- Replies: 3
- Views: 984
Re: Overall Order
Another instance where we would use the coefficients to come up with a rate law is if we're given the elementary steps of the reaction mechanism and we're told what the slow step is. But since that's not the case with the example given, again, we don't deal with coefficients.
- Mon Feb 16, 2015 6:46 pm
- Forum: General Rate Laws
- Topic: Writing rate law: the 2 questions
- Replies: 5
- Views: 1296
Re: Writing rate law: the 2 questions
In response to your first question, page 569 states that the rate law can only be determined from experimental data and cannot be determined from a chemical equation. I believe that means that regardless of what the coefficients are, you need to have experimental data to form a rate law and you woul...
- Mon Feb 16, 2015 6:35 pm
- Forum: General Rate Laws
- Topic: Overall Order
- Replies: 3
- Views: 984
Re: Overall Order
The overall order of a reaction is determined by adding the powers in a rate law expression. The coefficients are not used in determining rate laws such as those on page 569 ("The rate law for a reaction is experimentally determined and cannot in general be inferred from the chemical equation f...
- Mon Feb 16, 2015 5:39 pm
- Forum: First Order Reactions
- Topic: HW #14.35b
- Replies: 3
- Views: 981
Re: HW #14.35b
The ln10 comes from a modified version of the first-order half-life equation. Instead of using ln2 (which equals 0.693, the number we see in the half-life equation) which comes from the following analysis: ln\frac{[SO_{2}Cl_{2}]_{0}}{0.5\times [SO_{2}Cl_{2}]_{0}} = ln\frac{1}{0.5} = ln\frac{10}{5} =...
- Mon Feb 16, 2015 1:48 am
- Forum: General Rate Laws
- Topic: Finding the order of a reagent using method of initial rates
- Replies: 2
- Views: 623
Re: Finding the order of a reagent using method of initial r
If you look at page 56 in the course reader at the example problem with 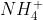 and
and 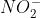 , we divide because we're finding the ratio of Rate 2 to Rate 1 (and this gives allows us to analyze the rates).
, we divide because we're finding the ratio of Rate 2 to Rate 1 (and this gives allows us to analyze the rates).
- Thu Feb 12, 2015 12:00 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: winter 2012 midterm question 3A
- Replies: 3
- Views: 995
Re: winter 2012 midterm question 3A
This is how I visualized finding W for BH2F. There's three possible states and only one molecule, so W = 3.
- Wed Feb 11, 2015 11:00 pm
- Forum: General Science Questions
- Topic: Combustion Reactions
- Replies: 3
- Views: 10151
Re: Combustion Reactions
For those who see this post but haven't found the answer, Chem_Mod answered it here: https://lavelle.chem.ucla.edu/forum/viewtopic.php?f=8&t=5148&sid=fdc826ba084c85c0219e9914a9020715 The phase of water is determined by the temperature at which the reaction occurs. Most reactions occur at roo...
- Sat Jan 31, 2015 6:27 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Does degeneracy = 0?
- Replies: 6
- Views: 1677
Re: Does degeneracy = 0?
It will always be greater than zero and at least 1.
- Sat Jan 31, 2015 6:02 pm
- Forum: Phase Changes & Related Calculations
- Topic: Calculating heat capacity from q=mCsΔT!
- Replies: 2
- Views: 988
Re: Calculating heat capacity from q=mCsΔT!
If the water and the metal weren't at the same temperature, there would still be heat flow from the metal (higher temperature) to the water (lower temperature), since heat is the energy transferred as a result of a temperature difference.
- Wed Jan 28, 2015 12:10 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units for specific heat capacity: kJ/gC or J/gC
- Replies: 3
- Views: 1476
Re: Units for specific heat capacity: kJ/gC or J/gC
They are basically the same units with different magnitude, so I would assume that either is fine, as long as you have the correct number of significant figures.
- Mon Jan 26, 2015 5:34 pm
- Forum: Phase Changes & Related Calculations
- Topic: Expansion done by/on the system
- Replies: 1
- Views: 638
Re: Expansion done by/on the system
Skatwal posted a good solution to this problem in response to another student's question here: viewtopic.php?f=130&t=5018 We can determine if work is done by or on a system by looking at the sign of w . If w is negative, the system is losing energy and expansion work is being done by the system....
- Mon Jan 26, 2015 12:59 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Self prep Question
- Replies: 1
- Views: 510
Re: Self prep Question
A few of our classmates have already asked this question. One of the original posts is linked here: https://lavelle.chem.ucla.edu/forum/viewtopic.php?f=76&t=5078&sid=38898720bd519479c315c42e4fa70642 . For the quizzes in the workbook, you may use the appendices in the textbooks. If a similar ...
- Sun Jan 25, 2015 12:41 pm
- Forum: Calculating Work of Expansion
- Topic: Sig Figs For Thermodynamic Problems?
- Replies: 2
- Views: 1325
Re: Sig Figs For Thermodynamic Problems?
If the number isn't used in the calculations, it isn't used in determining sig figs. We round off using sig figs because the measurements obtained are limited to the accuracy of the tools used to obtain them, so our answers can only be accurate for a certain amount of significant digits. If a number...
- Sat Jan 24, 2015 8:46 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Quiz 1 prep #1 Winter 2014 Question #11
- Replies: 2
- Views: 665
Re: Quiz 1 prep #1 Winter 2014 Question #11
In the first step, the system is expanding, so energy is lost to the surroundings in the form of work (the energy of the system is decreasing).
In the second step, we can assume the system is being compressed, so energy is being forced into the system (the energy of the system is increasing).
In the second step, we can assume the system is being compressed, so energy is being forced into the system (the energy of the system is increasing).
- Sat Jan 24, 2015 8:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Quiz 1 Winter 2014 #3
- Replies: 2
- Views: 737
Re: Quiz 1 Winter 2014 #3
The question asks for the enthalpy for the combustion of one mole of butane. The balanced reaction (with all whole number coefficients) is the combustion of two moles of butane, so you divide the enthalpy you get using the balanced reaction by two to get the answer for one mole . Yes, when you balan...
- Sat Jan 24, 2015 5:40 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Ungiven appendices
- Replies: 1
- Views: 1285
Re: Ungiven appendices
Another one of our classmates asked a similar question here: https://lavelle.chem.ucla.edu/forum/viewtopic.php?f=76&t=5078&sid=4c6a5f2ce3077b9f24614bdee4d51b6d . For the quizzes in the workbook, you may use the appendices in the textbooks. If a similar question is asked on the actual quiz, w...
- Sat Jan 24, 2015 2:50 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Does degeneracy = 0?
- Replies: 6
- Views: 1677
Re: Does degeneracy = 0?
I have no idea what the right answer to this would be, but I think the degeneracy is never going to be zero. If you consider a two-state system, the degeneracy is W=2^{n} . No matter what you plug in for n, you're never going to get to zero. If you plugged in zero, you would get a degeneracy of 1. Y...
- Sat Jan 24, 2015 12:07 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework Problem #7.75b
- Replies: 2
- Views: 551
Re: Homework Problem #7.75b
Bond enthalpies are calculated when all the substances are in the gas phase, but the problem has carbon in the solid phase (as graphite). The atomization of carbon is just the energy required to turn the graphite into gaseous carbon (it's essentially another way of saying sublimation), so all the su...
- Thu Jan 22, 2015 11:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Calculating the Net Change in Moles of a Gas to find Delta V
- Replies: 3
- Views: 5444
Re: Calculating the Net Change in Moles of a Gas to find Del
You can have a negative values. It follows the same sorta thinking as
values. It follows the same sorta thinking as  or
or 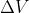 . It just means the change was a negative change and the final value is less than the initial value. So, for example, if there are 4 moles of gas in the reactants (instead of 2),
. It just means the change was a negative change and the final value is less than the initial value. So, for example, if there are 4 moles of gas in the reactants (instead of 2), 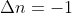 .
.
- Wed Jan 21, 2015 11:38 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Which value of R? Question 105
- Replies: 2
- Views: 2751
Re: Which value of R? Question 105
The units for work is joules. Using the 8.3145 J/K just allows some of the units to cancel and give us a final answer in joules. When deciding which value of R to use, just look to see what the question is asking and use the value that will allow you to use the least amount of steps for simplicity.
- Wed Jan 21, 2015 4:59 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Changes Based on Changes in Volume
- Replies: 2
- Views: 818
Re: Entropy Changes Based on Changes in Volume
The equation for entropy is always written the same way (without a negative sign -- to see why there is no negative sign in the equation, look back in your course reader or review the lecture for how the equation is derived): \Delta S = nRln\frac{V_{2}}{V_{1}} However, entropy can have a negative va...
- Mon Jan 19, 2015 10:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HW 7.101 Standard Enthalpies of Formation vs Bond Enthalpies
- Replies: 4
- Views: 1218
Re: HW 7.101
Remember that there are three ways to solve any enthalpy problem. Depending on the situation, you can use Hess' law, enthalpies of formation, or bond enthalpies. In this case, using the enthalpies of formation makes the most sense (since we aren't given multiple equations and their enthalpies to do ...
- Mon Jan 19, 2015 5:20 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Bond Enthalpies - #7.115
- Replies: 6
- Views: 1108
Re: Calculating Bond Enthalpies - #7.115
2 C_{8}H_{18} + 25O_{2} \rightarrow 18H_{2}O + 16 CO_{2} is the combustion reaction for octane, but since enthalpies are per mole , the reaction for which enthalpy is calculated is: C_{8}H_{18} + \frac{25}{2}O_{2} \rightarrow 9H_{2}O + 8 CO_{2} . So there's 8 CO 2 produced and you would divide the ...
- Sun Jan 18, 2015 11:07 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Self Test 7.6A How do we Find the Original Volume?
- Replies: 4
- Views: 2060
Re: Self Test 7.6A How do we Find the Original Volume?
Even though you don't need to find the exact initial volume for this problem, it's helpful to remember that since you're dealing with an ideal gas you can just use PV=nRT and the given initial conditions to find any unknown conditions. In this case, you're given P, T, and n (and you always have R). ...
- Sun Jan 18, 2015 8:33 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: HW #7.45, pg. 282
- Replies: 5
- Views: 1512
Re: HW #7.45, pg. 282
Yes, the specific heat capacity for air should be 1.01 J/C.g (then grams cancels out). This is mentioned in the Solution Manual Errors file linked here: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Solution_Manual_Errors_5Ed.pdf . There are also a few other errors in the solutio...
- Sun Jan 18, 2015 3:32 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Why internal energy is constant when temperature is constant
- Replies: 4
- Views: 1271
Re: Why internal energy is constant when temperature is cons
I also found this (http://www.eoht.info/page/Joule%E2%80%99s+second+law) online about Joule's second law. It states that ) (internal energy is a function of temperature). It follows the same logic as some of the other posts, if you feel like reading more into it.
(internal energy is a function of temperature). It follows the same logic as some of the other posts, if you feel like reading more into it.
- Sun Jan 18, 2015 3:24 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Derivation of Boltzwann equation for n moles of gas
- Replies: 3
- Views: 2565
Re: Derivation of Boltzwann equation for n moles of gas
All of this assumes two states. So we have S=k_{b}lnW as our equation. For 1 mole (N A ), W = 2^{N_{A}} \therefore S = k_{b}ln(2^{N_{A}}) We use the log rule to get N_{A}k_{b}ln(2) N_{A} \times k_{b} = R , so we get Rln(2) This is for one mole, so if we have n moles, we just ...
- Sat Jan 17, 2015 5:02 pm
- Forum: Phase Changes & Related Calculations
- Topic: Homework Problem #19
- Replies: 5
- Views: 1298
Re: Homework Problem #19
It's not necessarily saying that the heat gained by the water is negative. It just means that the heat lost and heat gained are equal and opposite.
- Sat Jan 17, 2015 3:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reaction Enthalpies - #7.53
- Replies: 3
- Views: 724
Re: Reaction Enthalpies - #7.53
I'm going to assume you're looking at the solutions manual. -20,000J just comes from the previous step, where the enthalpy per mole of zinc consumed is determined and then multiplied by the number of moles of zinc. 8.5g Zinc releases 20 kJ and this is equivalent to 20,000 when converted to joules.
- Thu Jan 15, 2015 11:01 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: p 240 Self Test 7.1B
- Replies: 3
- Views: 1302
Re: p 240 Self Test 7.1B
The gases in the four cylinders of an automobile engine expand from 0.22 L to 2.2 L during one ignition cycle. Assuming that the gear train maintains a steady pressure of 9.60 atm on the gases, how much work can the engine do in one cycle? You can follow the same steps as Sample 7.1 on the previous ...
- Thu Jan 15, 2015 1:40 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HW 51
- Replies: 3
- Views: 701
Re: HW 51
\Delta H values are determined by taking the difference in enthalpies of the products and reactants, which are both measured at 298 K (which is a stadard condition, 25^{\circ }C ). You can just assume that is the temperature when given a \Delta H value. (There is a note about this on pg. 261 of the...
- Thu Jan 15, 2015 1:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 7.15 clarifications
- Replies: 5
- Views: 1084
Re: 7.15 clarifications
Justin,
Wouldn't open beakers (in the diagrams) indicate open systems that are exposed to the atmosphere and an implied 1 atm of external pressure?
Even then, I think Skatwal's explanation is the best way to approach this problem. Using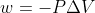 just seems too complicated.
just seems too complicated.
Wouldn't open beakers (in the diagrams) indicate open systems that are exposed to the atmosphere and an implied 1 atm of external pressure?
Even then, I think Skatwal's explanation is the best way to approach this problem. Using
- Thu Jan 15, 2015 12:28 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 7.15 clarifications
- Replies: 5
- Views: 1084
Re: 7.15 clarifications
Hey Martha, Could you clarify how you got your \Delta V values? In part a, you used T_{f} < T_{i} making \Delta V negative (but temperature is constant so wouldn't T_{f}=T_{i} ?). Then in part b, you said \Delta V is positive, since there are more liquid particles than gas particles (how did you com...
- Wed Jan 14, 2015 11:39 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law, Multiplying Coefficients
- Replies: 2
- Views: 8651
Re: Hess's Law, Multiplying Coefficients
Problem 7.63 deals with the \Delta H^{\circ }_{c}(H_{2}, g) which is the enthalpy of combustion for one mole of H_{2} . (This is the same equation and enthalpy as the reaction for the formation of one mole of water, but since the problem specifically deals with the enthalpy of combustion, I ...
- Tue Jan 13, 2015 2:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law Addition?
- Replies: 3
- Views: 804
Re: Hess's Law Addition?
The problem gives you the final reaction (the one we are trying to find \Delta H for). Then it gives you the \Delta H_{c}^{\circ} of two hydrocarbons and hydrogen gas. This value tells us the \Delta H for the combustion reactions for each of the hydrocarbons and/or hydrogen gas. So, for example, whe...
- Tue Jan 13, 2015 1:37 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question 7.27, Gas constant temperature units
- Replies: 9
- Views: 2283
Re: Question 7.27, Gas constant temperature units
R is the gas constant. It can be found online, on the back of the periodic table that came with the course reader, and in your textbook.
- Sat Jan 10, 2015 9:10 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Are all combustions endothermic?
- Replies: 2
- Views: 9624
Re: Are all combustions endothermic?
Recall Dr. Lavelle's example of an endothermic reaction: melting ice. Ice melts because it takes the energy from its surroundings; that's why water gets cooler when it has ice melting in it. The same kind of thing doesn't happen when you combust something; the surroundings aren't getting colder. Com...
- Sun Dec 14, 2014 3:56 am
- Forum: *Titrations & Titration Calculations
- Topic: Multistep Titration Calculations Explained
- Replies: 2
- Views: 2179
Re: Multistep Titration Calculations Explained
Additional Note: - Be sure to use the 5% rule to validate your assumption each time you ignore the +X or –X when dealing with the concentrations. There were two other times in this problem where we should have used the 5% rule, but we omitted them for the sake of time. Corrections to the Video: - @ ...

