Search found 11 matches
- Sat Mar 12, 2016 3:02 pm
- Forum: *Carboxylic Acids
- Topic: Carboxyl group and parent chain
- Replies: 1
- Views: 1396
Carboxyl group and parent chain
When naming a carboxylic acid, do you count the carbon of the carboxyl group as part of the parent chain?
- Mon Mar 07, 2016 3:33 pm
- Forum: *Amines
- Topic: Charge of amine
- Replies: 1
- Views: 1418
Charge of amine
Why does a quaternary amine have a +1 charge?
- Mon Mar 07, 2016 3:22 pm
- Forum: *Alkanes and Substituted Alkanes (Staggered, Eclipsed, Gauche, Anti, Newman Projections)
- Topic: Bond angle strain
- Replies: 1
- Views: 469
Bond angle strain
What is bond angle strain and how does it affect conformations?
- Sun Feb 28, 2016 7:55 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Pseudo-equilibrium constant
- Replies: 1
- Views: 430
Pseudo-equilibrium constant
Would you calculate the pseudo-equilibrium constant for a reaction the same way you would a normal equilibrium constant? And does each transition state in a reaction have its own pseudo-equlilibrium constant?
- Thu Feb 18, 2016 11:25 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Calculate rate constant given temp
- Replies: 1
- Views: 447
Calculate rate constant given temp
What would be the equation/formula to use to calculate the rate constant of a rxn given the temperature?
- Thu Feb 11, 2016 9:58 pm
- Forum: Zero Order Reactions
- Topic: Saturation of catalysts
- Replies: 1
- Views: 913
Saturation of catalysts
Is a zero order rxn the only type of rxn whose rate is affected by the level of saturation of a catalyst?
- Fri Feb 05, 2016 1:32 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 4466263
Re: Chemistry Jokes
What is Kylo Ren's favorite type of reaction?
A First Order reaction.
A First Order reaction.
- Fri Feb 05, 2016 1:01 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: sign of k
- Replies: 1
- Views: 559
sign of k
Why can the rate constant (k) for a first order rxn only be positive?
- Thu Jan 28, 2016 3:33 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G for a system not at equilibrium
- Replies: 1
- Views: 551
G for a system not at equilibrium
For the equation 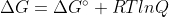 do you calculate Q using the concentrations of the product and reactants at that particular point in the reaction?
do you calculate Q using the concentrations of the product and reactants at that particular point in the reaction?
- Thu Jan 21, 2016 4:15 pm
- Forum: Calculating Work of Expansion
- Topic: Expansion against constant pressure
- Replies: 2
- Views: 692
Expansion against constant pressure
Is expansion against constant pressure in a system reversible? Would it be considered reversible in the case of an infinite number of steps?
- Fri Jan 15, 2016 10:13 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Work in a system
- Replies: 1
- Views: 476
Work in a system
Is a change in volume in a system the only way that energy is exchanged as work?

