Search found 32 matches
- Wed Mar 09, 2016 10:29 am
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Course Reader: 3,6-Dimethyl-3-Octene
- Replies: 1
- Views: 594
Re: Course Reader: 3,6-Dimethyl-3-Octene
You are essentially right when talking about priority for naming Z and E. Lets start with the right side first The carbon on the right is connected to a hydrogen (not shown) and the rest of the carbon chain. Since the carbon is angled down, the hydrogen must be pointed up. Also, since carbon has a b...
- Tue Mar 08, 2016 9:32 pm
- Forum: *Haloalkenes
- Topic: (E)-3-Chloro-4-fluoro-7-methyloct-3-en-5-yne
- Replies: 3
- Views: 1648
Re: (E)-3-Chloro-4-fluoro-7-methyloct-3-en-5-yne
Reading over my answer, I can I wasn't very clear. Your are right in that double and triple bonds have priority over substituents in all cases. Also double and triple bonds have equal priority. As such, in this example, prioritize the the double bond is given the lower number, because it give the su...
- Tue Mar 08, 2016 9:27 pm
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Assigning Priority to Atoms/Atoms Groups
- Replies: 1
- Views: 728
Re: Assigning Priority to Atoms/Atoms Groups
Based on this molecule, Cl actually has priority. Notice that the name of this compound is (E). This means that the structure is a trans, and that the bigger elements attached to the carbons in the double bond are on opposite sides. If we look at the carbon on the very right, we compare Cl and CH2CH...
- Thu Mar 03, 2016 6:59 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Relationship b/w A and k (Arrhenius)
- Replies: 1
- Views: 465
Re: Relationship b/w A and k (Arrhenius)
The Arrhenius equation is:
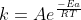
As you increase the value of A, k increases.
As you increase the value of A, k increases.
- Tue Mar 01, 2016 11:32 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: number 30 chapter 4 homework
- Replies: 1
- Views: 510
Re: number 30 chapter 4 homework
In the Introduction to Organic Chemistry, on pg. 154 the book says "reactions with activation energies below 80 kj/mol can occur at room temperature." Question number 30 is just some organic chemistry concept, there is no math involved from what I can tell.
- Tue Mar 01, 2016 11:28 pm
- Forum: *Cycloalkenes
- Topic: Naming Order
- Replies: 2
- Views: 716
Re: Naming Order
Priority goes to the double and triple bonds when numbering and when naming. When both double and triple bonds are present, I believe that double bonds get priority.
- Mon Feb 29, 2016 10:41 am
- Forum: *Haloalkenes
- Topic: (E)-3-Chloro-4-fluoro-7-methyloct-3-en-5-yne
- Replies: 3
- Views: 1648
Re: (E)-3-Chloro-4-fluoro-7-methyloct-3-en-5-yne
You are right in thinking that in a normal carbon chain a triple bond would get priority over a double and single bond. However, recall that when naming structures and assigning numbers we have to look at the numbering of the substituents first. Since we give chlorine and fluorine the smaller number...
- Sat Feb 27, 2016 3:18 pm
- Forum: *Alkenes
- Topic: Numbering for Cycloalkanes
- Replies: 2
- Views: 611
Re: Numbering for Cycloalkanes
The example in the course reader and the example Lavelle said in class were for different types of organic compounds. Let's first start with the book. The compound in the course reader is called 4-Ethyl-2-Methyl-1-Propycyclohexane rather than 1-Ethyl-3-Methyl-4-Propylcyclohexane because the first na...
- Thu Feb 18, 2016 4:14 pm
- Forum: General Rate Laws
- Topic: Finding the rate of formation of a reaction 2006MT
- Replies: 2
- Views: 1109
Re: Finding the rate of formation of a reaction 2006MT
Since both O2 and NO2 are products, we know that they are being formed, so the rates for both species will be positive. O2 has a rate of 2.28 and and since NO2 to O2 is a 4:1 ratio, if we multiply 2.28 by 4 we get 9.12. 4 NO2 are being formed for each 1 of O2, or the rate of NO2 is 4 times that of O...
- Fri Feb 12, 2016 5:55 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Intermediate
- Replies: 1
- Views: 431
Re: Intermediate
Intermediates are only in reactions that have multiple parts. Given a reaction that satisfies such conditions, we can identify something as an intermediate if we see it formed in one step and then used in another step. In other words, the intermediate is not a reactant or product because it doesn't ...
- Sat Feb 06, 2016 8:19 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Question 14.41 finding Q
- Replies: 1
- Views: 429
Re: Question 14.41 finding Q
The first step we need to do is too write the the full reaction from the cell diagram. We know that the anode is on the left and the cathode is on the right so the 2 half reations are: Anode: Cu_{s} \rightarrow Cu^{2+} + 2e^{-} Cathode: Cu^{2+} + 2e^{-} \rightarrow Cu_{s} Combining these 2 equations...
- Fri Feb 05, 2016 9:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: k>1
- Replies: 1
- Views: 475
Re: k>1
Remember from the Nerst Equation: E=E^{0}-\frac{RT}{nF}*ln(Q) and that at equilibrium E^{0}=\frac{RT}{nF}*ln(K) and solving for K, when we change ln to log, we get K=10^{\frac{n*E^{0}}{0.0592}} Since n is always a positive, the only thing that matters is the cell potential. If the Ca...
- Fri Feb 05, 2016 9:16 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: H+ in Galvanic Cell Diagrams
- Replies: 1
- Views: 1052
Re: H+ in Galvanic Cell Diagrams
Since Hydrogen has a charge and ends up affecting the placement of electrons for the half reaction, it is written in the cell diagram.
H2O isn't reduced or oxidized and because it has no charge it isn't mentioned. H+ isn't reduced or oxidized, but it affects the charge.
H2O isn't reduced or oxidized and because it has no charge it isn't mentioned. H+ isn't reduced or oxidized, but it affects the charge.
- Mon Feb 01, 2016 5:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy and Temperature
- Replies: 1
- Views: 563
Re: Internal Energy and Temperature
The simple definition of energy is the ability to do work. In class we talked about 3 main ways to change energy: 1. Add or remove a substance 2. Heat or Cool a substance 3. Do work on the system If a substance cools, its motion of particle slows down and it loses energy. And if a substance heats up...
- Fri Jan 29, 2016 8:36 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Salt Bridge
- Replies: 1
- Views: 481
Re: Salt Bridge
In a normal battery an anode and cathode is present. The flow of electrons goes from anode to cathode making the anode side very positive and the cathode side very negative. In a short amount of time, less than a few seconds, the anode side gets such a positive gradient that electrons have trouble e...
- Sun Jan 24, 2016 11:30 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Negative Reaction Entropies for Ions
- Replies: 1
- Views: 526
Re: Negative Reaction Entropies for Ions
First note that the idea that entropy of the universe is always increasing, not necessarily the system. When we do a reaction we can define a system as having "0 entropy" and the process the system goes through can either decrease of increase this arbitrary 0 value. For example, freezing w...
- Sat Jan 16, 2016 5:26 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs. Irreversible Processes
- Replies: 2
- Views: 757
Re: Reversible vs. Irreversible Processes
An irreversible reaction is where the external pressure will always be constant. As such we can use w=-PdV, because the force needed to move something will always be pushing against a constant pressure. This is irreversible because the process can only expand one way: from high pressure toward the a...
- Fri Jan 08, 2016 7:37 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Intensive properties
- Replies: 2
- Views: 3471
Re: Intensive properties
Intensive properties are properties that don't change if the sample size change. Specific heat capacity and molar heat capacity are intensive because in the equations, you divide by the amount of concentration of the substance giving you a per mol or per gram value. For example, if you have a little...
- Sat Dec 05, 2015 2:35 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: M.O
- Replies: 1
- Views: 623
Re: M.O
When your nuclear charge (the z value, also known as the atomic value) is less than 8 the energy difference between the pi is sigma is so close to each other that the pi is filled first. As you go across the periodic table and down groups, the energy difference between sigma and pi grows, and sigma ...
- Thu Dec 03, 2015 4:33 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Depronatation
- Replies: 1
- Views: 559
Re: Depronatation
The Equation for Benzoic Acid is : C_{6}H_{5}COOH + H_{2}O \rightleftharpoons C_{6}H_{5}COO^{-} + H_{3}O^{+} When Hydrochloric Acid is added, which I'm assuming is the question, there is a 100% disassociation, meaning that HCl splits into a proton and Cl-. The proton normally would increase the conc...
- Wed Nov 25, 2015 8:48 pm
- Forum: *Titrations & Titration Calculations
- Topic: Reduction of Acid 12.19
- Replies: 3
- Views: 3228
Re: Reduction of Acid 12.19
Remember that HCl is a strong acid so it completely dissociates. This mean thats no there is no need for an ice box because whatever the original concentration of HCl is, the value will go to zero. For example if you have 0.1M of HCl you will get .1M of H30+ If you reduce the concentration by 12%, y...
- Sat Nov 21, 2015 9:04 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: When to use Ka1 and Ka2?
- Replies: 1
- Views: 27199
Re: When to use Ka1 and Ka2?
The majority of polyprotic acids (ones that have multiple protons, or Hydrogens) are considered weak because their Ka2 values are extremely small. For example if we look at H2CO3, the Ka1 value is 10^-7 (which is not that big), however its Ka2 value is 10^-11. Relatively the second value doesn't imp...
- Sat Nov 14, 2015 11:29 pm
- Forum: Naming
- Topic: Iron vs ferrate & oh2
- Replies: 3
- Views: 1049
Re: Iron vs ferrate & oh2
When writing the the name of the compound, aqua is always used for H2O, however water is written as OH2 to show that the oxygen bonds to the transition metal, and not the hydrogen atom. In addition the suffix -ate is added when the coordination compound has a negative charge, as in it is an anion. F...
- Sat Nov 07, 2015 5:20 pm
- Forum: Photoelectric Effect
- Topic: Work Function vs De Broglie
- Replies: 4
- Views: 4905
Re: Work Function vs De Broglie
The energy of a photon is is Planks constant (h) times the frequency (v), which is equal to E=hv. Work function is just the minimum amount of energy required to remove an electron, also known as threshold energy. In other words, the formula you mention is exactly the same thing as hv=Ek + work funct...
- Mon Nov 02, 2015 11:05 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Homework Problem 4.63a
- Replies: 2
- Views: 659
Re: Homework Problem 4.63a
B2 has 6 electrons that take place in the molecular orbital, 3 from each Boron atom. The first 4 electrons are used in the 2s sigma bond and the 2s sigma anti-bond. This leaves 2 electrons that need to be placed in the molecular orbital. Also, because Boron has a z value of less than 8, the pi orbit...
- Sat Oct 31, 2015 12:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Phosphate Lewis Dot structure
- Replies: 1
- Views: 4217
Re: Phosphate Lewis Dot structure
The Phosphate ion is written as PO_{4}^{-3} . This means that there are a total of 32 valence electrons for bonding. The central atom is is Phosphorus because it has a lower ionization energy. Surrounding it with all 4 oxygen, we use 32 electrons. However, calculating the formal charge we find out t...
- Sat Oct 24, 2015 6:37 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Question 4.25 Polarity of CH2Cl2
- Replies: 1
- Views: 1212
Re: Question 4.25 Polarity of CH2Cl2
The problem with the lewis structure is that it shows a 2D image, when in reality the molecule is 3D. With the tetrahedral structure, all the non-central atoms will be 109.5 degrees away from each other, so no matter where you draw the Chlorine atoms, they will technically always be next to each oth...
- Sat Oct 24, 2015 6:25 pm
- Forum: Octet Exceptions
- Topic: HW 3.59
- Replies: 1
- Views: 476
Re: HW 3.59
Although the way you describe would technically give the structure a lower formal charge, you have to recall that Nitrogen is part of the 2nd group and thus must obey the octet rule because it does not have access to the 3d sub-shell. If Nitrogen double bonds it would be sharing 10 electrons which i...
- Sat Oct 24, 2015 4:58 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 1
- Views: 372
Re: Bond Angles
Remember that bond angles for the molecules are based off the real structures, which will be 3D. When drawing lewis structures, we can only diagram the 2D of how the molecule will share its electrons, not how they will be shaped/what the bong angles are. The bond angles for structures just have to m...
- Mon Oct 12, 2015 10:39 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Chapter 2 Question 27
- Replies: 1
- Views: 484
Re: Chapter 2 Question 27
Remember that the angular quantum number, l , relates to the shape and can range from 0 to n-1. When l is equal to 0, this is the s-oribtal, or spherical shape which only has 1 orbital. When l is equal to 1, this is the p-oribtal, or the double-lobe which can have 3 different orbitals. When l is equ...
- Wed Oct 07, 2015 9:25 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Hund's rule-Carbon
- Replies: 1
- Views: 771
Re: Hund's rule-Carbon
The electron configuration for carbon can be written both ways, as both notations are correct. When drawing out the electron configuration the first notation is technically correct as it demonstartes that electrons will occupy all the sub-shells before pairing up with each other, however the second ...
- Sat Oct 03, 2015 11:59 am
- Forum: Photoelectric Effect
- Topic: Problem 1.33 part c
- Replies: 1
- Views: 568
Re: Problem 1.33 part c
For part c, we use the formula for the photoelectric effect: E(photon) - \Phi = E(kinetic) Since part A gave us the velocity of the ejected electron we can find the E(kinetic) with E(k) = \frac{1}{2}m^{2} v^{2} , with m being the mass of the electron and v being the given velocity. In part B we solv...

