Search found 20 matches
- Fri Mar 11, 2016 10:34 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Why is heat of vaporization not considered in this problem?
- Replies: 2
- Views: 456
Re: Why is heat of vaporization not considered in this probl
Yes! Since it is required to boil it is just the amount of heat needed to get to boiling point. Vaporization would occur at anything above 100 degrees celsius the difference comes from the wording, if it had said vaporize we would use the enthalpy of vaporization but since it says to boil we can om...
- Fri Mar 11, 2016 1:45 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Why is heat of vaporization not considered in this problem?
- Replies: 2
- Views: 456
Why is heat of vaporization not considered in this problem?
In Q1A of the 2011 Final, it asks for us to calculate the amount of methane needed to combust to generate enough heat to boil 50.0mL of water. However, in the solutions it does not consider the amount of energy required to vaporize the water - it only considers the energy to raise the temperature to...
Re: Naming
Keep in mind that triple bonds have priority for lowest numbering in alkynes
- Sun Mar 06, 2016 8:11 pm
- Forum: *Cycloalkanes
- Topic: Numbering Carbons
- Replies: 3
- Views: 809
Re: Numbering Carbons
Double and triple bonds have priority over substituents and should always be given the lowest possible number. For substituents, you give priority to them based on alphabetical order. Alphabetical order does not include any of the prefixes (di, tri, etc.) and it does not include the common prefixes...
- Sun Mar 06, 2016 8:05 pm
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Cis and trans vs Z and E
- Replies: 1
- Views: 754
Cis and trans vs Z and E
How do we know when to use each classification? When do we know whether or not we should classify according to cis and trans or z and e?
- Wed Mar 02, 2016 2:52 pm
- Forum: *Alkanes
- Topic: Prioritizing in naming
- Replies: 2
- Views: 561
Prioritizing in naming
How come for 4-ethyl-2,2-dimethyl-hexane dimethyl is prioritized for lower numbering? Shouldn't ethyl be prioritized so that it would be 3-ethyl-5,5-dimethyl-hexane because ethyl is listed first in the name?
- Thu Feb 18, 2016 2:55 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-equilibrium vs steady-state
- Replies: 2
- Views: 1186
Pre-equilibrium vs steady-state
How do we know which of these two methods to use? Are there specific conditions we should look for or will the question tell us which of the two to use?
- Mon Feb 08, 2016 10:51 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: How to know what units for pressure to use?
- Replies: 2
- Views: 521
Re: How to know what units for pressure to use?
But the units don't cancel out? It's 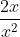
- Mon Feb 08, 2016 10:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagrams and spectator ions
- Replies: 1
- Views: 582
Cell diagrams and spectator ions
Are we supposed to include spectator ions when we draw the cell diagrams in line notation? Online sources say that you shouldn't but the solution for 14.15 says that you should.
- Mon Feb 08, 2016 5:51 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: How to know what units for pressure to use?
- Replies: 2
- Views: 521
How to know what units for pressure to use?
For question 4 on the 2011 practice midterm, the answer was given in bar even though there was no indication of it being used in the constants provided. How do we know to use bar rather than kPa or atm?
- Sun Feb 07, 2016 7:27 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: How to identify systems
- Replies: 2
- Views: 571
Re: How to identify systems
An isolated system is similar to a calorimeter with a lid. None of the matter inside will come out, and the calorimeter will contain any transfer of energy between the system and its surroundings. An closed system is like a lightbulb. It is sealed so that none of the components can come out but can ...
- Mon Jan 25, 2016 10:57 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Isothermal, reversible expansion at constant pressure
- Replies: 1
- Views: 349
Isothermal, reversible expansion at constant pressure
Which work equation should we use for problems with isothermal, reversible reactions at constant pressure? The course reader says to use 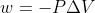 for reversible expansions with constant pressure and
for reversible expansions with constant pressure and ) for isothermal, reversible expansions with variable pressure.
for isothermal, reversible expansions with variable pressure.
- Sat Jan 23, 2016 4:49 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Standard entropy of vaporization of ammonia
- Replies: 2
- Views: 1259
Re: Standard entropy of vaporization of ammonia
So would we assume that the pressure has decreased after the ammonia has become a gas?
- Thu Jan 21, 2016 10:51 am
- Forum: Phase Changes & Related Calculations
- Topic: Diatomic vs Monotomic Gases Clarification
- Replies: 2
- Views: 580
Re: Diatomic vs Monotomic Gases Clarification
Diatomic molecules will tend to have higher entropy due to this equation: S = k_{b}lnW The number of microstates (W) is given by the equation: W = m^{n} m is the number of states while n is the total number of particles. A diatomic molecule would have a greater number of microstates and, therefore, ...
- Thu Jan 21, 2016 10:42 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Standard entropy of vaporization of ammonia
- Replies: 2
- Views: 1259
Standard entropy of vaporization of ammonia
Why is it that ammonia can remain in a gaseous state while below the temperature of vaporization? For question 9.20, we are supposed to calculate the standard entropy of vaporization of ammonia @210K. The temperature of vaporization of ammonia is 329.4K. To do the problem, we have to assume the liqu...
- Mon Jan 11, 2016 9:38 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Using ideal gas constants
- Replies: 1
- Views: 370
Using ideal gas constants
How do we know when or what gas constants to use when calculating for the change in internal energy of a gas with constant pressure? For example, 8.9 from the textbook uses 8.314 and 0.08206, but how did they know to use these constants?
- Mon Nov 23, 2015 12:31 am
- Forum: Empirical & Molecular Formulas
- Topic: Video: Empirical Formulas
- Replies: 3
- Views: 907
Video: Empirical Formulas
Here's my video on how to find the empirical formula of a compound. Contributors include: Anthony Giron, Carrie Huang, and Daniel Zhang.
- Sun Nov 08, 2015 8:42 pm
- Forum: Lewis Structures
- Topic: Formal charge vs octet rule
- Replies: 2
- Views: 808
Formal charge vs octet rule
Does the formal charge or the octet rule create a more stable Lewis structure for elements who can have expanded octets? For example, sulfur in SO _{2} can form a double bond with both oxygens to have a low formal charge. However, sulfur would have an expanded octet. If sulfur formed a double bond w...
- Tue Oct 13, 2015 8:34 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration for Tungsten (W)
- Replies: 1
- Views: 2949
Electron Configuration for Tungsten (W)
Why is the electron configuration for tungsten  instead of
instead of 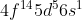 ? Shouldn't the 6s orbital lend an electron to the 5d orbital for a more stable half-orbital?
? Shouldn't the 6s orbital lend an electron to the 5d orbital for a more stable half-orbital?
This is based on the answer for 2.43 from the textbook, part e).
This is based on the answer for 2.43 from the textbook, part e).
- Sun Oct 11, 2015 11:58 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg vs. Bohr equation for hydrogen atom
- Replies: 1
- Views: 4522
Re: Rydberg vs. Bohr equation for hydrogen atom
You can use both equations. If using the Rydberg you can use the frequency value to find E with 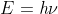
You can plug this into the Rydberg formula to figure out the principle quantum level the electron started from.
You can plug this into the Rydberg formula to figure out the principle quantum level the electron started from.

