Search found 12 matches
- Sat Mar 12, 2016 9:33 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nerst Equation?
- Replies: 1
- Views: 653
Re: Nerst Equation?
I believe Professor Lavelle mentioned that it is derived from not only simplifying the constants, but also multiplying by a factor which converts lnQ to logQ
- Sat Feb 27, 2016 11:59 pm
- Forum: *Alkanes
- Topic: Tert- and Sec- Prefixes
- Replies: 2
- Views: 546
Re: Tert- and Sec- Prefixes
I believe it's because the carbons on the parent chain aren't counted for the substituent chain
- Sat Feb 20, 2016 6:14 pm
- Forum: First Order Reactions
- Topic: Quiz #2 Question #1 on Thursday
- Replies: 1
- Views: 554
Re: Quiz #2 Question #1 on Thursday
You know that the substance is a first order, since the amount of the substance does not change the half life (50 days regardless of how much you start with.) Since you know it's a first order, I believe you can simply keep dividing in half until you reach 3g OR Use half life = 0.693/k and solve for...
- Sat Feb 13, 2016 8:47 pm
- Forum: General Rate Laws
- Topic: Integrated vs. Differential Rate Laws
- Replies: 1
- Views: 510
Re: Integrated vs. Differential Rate Laws
I believe you can use an integrated rate law to solve for initial concentration if you aren't already given it. You can also use the integrated rate law to draw a graph which tells you the order of the reaction. For example, if the graph of ln[A] vs. t is a straight line, you know you have a first o...
- Sat Feb 06, 2016 10:34 pm
- Forum: First Order Reactions
- Topic: Changing Differential Rate Law to Integrated Rate Law
- Replies: 1
- Views: 424
Re: Changing Differential Rate Law to Integrated Rate Law
a refers to the coefficient in a general equation 
The term -1/a defines how fast A is being consumed relative to the other reactants and products in the equation. For example B may not be consumed at the same rate A does, it's rate of consumption is dependent on b.
The term -1/a defines how fast A is being consumed relative to the other reactants and products in the equation. For example B may not be consumed at the same rate A does, it's rate of consumption is dependent on b.
- Sun Jan 31, 2016 10:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Example 14.3 moles confusion? (Not HW question, example.)
- Replies: 1
- Views: 422
Re: Example 14.3 moles confusion? (Not HW question, example.
n is the number of moles of electrons transferred. When the redox reaction between zinc and copper are balanced there are 2 moles of electrons transferred.

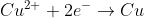
- Sun Jan 24, 2016 10:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy of atomization
- Replies: 1
- Views: 1815
Re: Enthalpy of atomization
I believe the enthalpy of atomization is the enthalpy change associated with breaking apart a molecule into it's individual substances.
For example, the enthalpy of atomization for H2O would be the sum of the bond enthalpies to split the molecule into it's individual atoms.
For example, the enthalpy of atomization for H2O would be the sum of the bond enthalpies to split the molecule into it's individual atoms.
- Sun Jan 17, 2016 11:18 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework 8.49 question
- Replies: 3
- Views: 1024
Re: Homework 8.49 question
You find w using w=-P * delta V which can be derived from the ideal gas equation. PV=nRT P\Delta V = \Delta n RT -P\Delta V =- \Delta n RT Substitute -P\Delta V for - \Delta n RT gives you w=-\Delta nRT . New equation is \Delta U=q + (-\Delta nRT) where q is given, R is a constant, T=298K, a...
- Sun Jan 10, 2016 5:12 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question regarding Enthalpy
- Replies: 2
- Views: 1818
Re: Question regarding Enthalpy
Yes it is true, I like to think of this in terms of a reaction profile. For example, say the reactants have a lower energy than their products. That means that the reaction is endothermic and requires energy. However, once you switch them, and go from products to reactants, you're now going from a h...
- Sun Nov 22, 2015 12:09 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Video: Molecular Orbital Diagrams and Configurations
- Replies: 3
- Views: 672
Video: Molecular Orbital Diagrams and Configurations
A video explaining how to fill molecular orbital diagrams
- Sun Oct 25, 2015 9:46 pm
- Forum: Hybridization
- Topic: Shape
- Replies: 2
- Views: 518
Re: Shape
Correct me if I'm wrong but, molecular shape is determined by the orientation of the atoms and regions of electron density. With resonance, the bonds are shifted around, but the number of electron density regions remains the same, so the orientation of the atoms and the molecular shape of the atoms ...
- Sat Oct 10, 2015 11:12 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Chromium, Copper, etc.
- Replies: 2
- Views: 628
Re: Chromium, Copper, etc.
For elements such as Chromium or Copper, they will have a filled or half filled d orbital. Electrons prefer to be in either a half filled or full d-orbital because it is more stable due to symmetry. For example, Copper will have an electron configuration of [Ar]3d 10 4s 1 because it is more stable w...

