Search found 30 matches
- Sun Mar 13, 2016 8:05 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Winter 2013 Final Question 3A
- Replies: 3
- Views: 701
Re: Winter 2013 Final Question 3A
Yes the Half reaction values are given to us. They solved it in 2 ways in that problem. If you are using the formula Cathode - Anode you just plug the positive values (reductions) that you are given on the sheet directly into that formula without changing the sign because the negative in the formula...
- Sun Mar 13, 2016 7:44 am
- Forum: Balancing Redox Reactions
- Topic: Cell Diagrams
- Replies: 4
- Views: 942
Re: Cell Diagrams
The Pt(s) are going to be acting as anodes or cathodes that don't affect the solutes and concentrations, they will be placed on either side of the cell diagram as demonstrated above.
- Sun Mar 13, 2016 7:24 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Winter 2011 Final Question
- Replies: 2
- Views: 624
Re: Winter 2011 Final Question
In addition to the Boltzmann equation, you can think about the 2 molecules stacking into a crystal N-0 can stack like this: N-ON-O N-OO-N N-ON-O or it can mix O-NN-O this possibility for mixing contributes to residual entropy at Ok N-ON-O N-ON-O Whereas BF3 doesn't have different possible stackings ...
- Sat Mar 12, 2016 7:39 pm
- Forum: *Alkanes
- Topic: Priority naming - Alphabetical vs. # substituents
- Replies: 1
- Views: 460
Priority naming - Alphabetical vs. # substituents
If you have, ex. 3 methyl groups and one chlorine, would you apply the lower number to the trimethyl since there are more of them or the chloro because it comes first alphabetically?
ex. 4-chloro-2,2,4-trimethylpentane
or 2-chloro-2,4,4-trimethylpentane
Thanks!
ex. 4-chloro-2,2,4-trimethylpentane
or 2-chloro-2,4,4-trimethylpentane
Thanks!
- Fri Mar 11, 2016 2:51 pm
- Forum: *Alkanes and Substituted Alkanes (Staggered, Eclipsed, Gauche, Anti, Newman Projections)
- Topic: Winter 2014 final question 7 part F newman projections
- Replies: 3
- Views: 1077
Re: Winter 2014 final question 7 part F newman projections
How would we know that the chlorine is bigger than the methyl group when arranging the second most stable conformation?
- Sun Mar 06, 2016 7:05 pm
- Forum: *Alkanes
- Topic: Vinyl and allyl groups
- Replies: 1
- Views: 654
Vinyl and allyl groups
Will vinyl groups ever be used in IUPAC naming or are they just used to distinguish a particular halogen? What is the effect of their distance from the double bond? Does this significantly alter a halogen's effect on the molecule if it is allyl vs. vinyl?
- Sun Feb 28, 2016 10:55 pm
- Forum: *Complex Reaction Coordinate Diagrams
- Topic: Reaction Profile Delta G
- Replies: 4
- Views: 2056
Reaction Profile Delta G
In a 2 step reaction, the reaction profile would have two transition states. In the process of figuring out which has a larger delta G, would you measure the difference between the top of both the first and the second transition states to the initial reaction delta G value? Or would you compare TS1 ...
- Fri Feb 19, 2016 9:15 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: M Calculations
- Replies: 1
- Views: 594
Re: M Calculations
If you are given a reaction: aA + bB = cC you can use this formula to compare them: -\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=\frac{1}{c}\frac{d[C]}{dt} The first two are negative because they are reactants, they are decreasing to form the positive product. If the coefficient of a is o...
- Tue Feb 09, 2016 9:47 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Winter 2015 Midterm Q1 heat
- Replies: 1
- Views: 522
Re: Winter 2015 Midterm Q1 heat
Water is liquid at 22 degrees as well as 25 degrees, it uses the same heat capacity from 0-100 degrees, you do not need to split this portion into steps.
- Tue Feb 09, 2016 9:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: Intensive/Extensive
- Replies: 5
- Views: 1259
Re: Intensive/Extensive
- Tue Feb 09, 2016 9:07 pm
- Forum: Phase Changes & Related Calculations
- Topic: Thursday Quiz 1, Question 3; See post for question
- Replies: 3
- Views: 675
Re: Thursday Quiz 1, Question 3; See post for question
Yes, your answer is negative because you are dealing with the heat removed from the gas, each step will be negative and they will all be added together.
- Tue Feb 09, 2016 8:53 pm
- Forum: Phase Changes & Related Calculations
- Topic: 2014 Midterm Question 5C
- Replies: 2
- Views: 710
Re: 2014 Midterm Question 5C
E is not a state function: see this question
viewtopic.php?f=140&t=3754
viewtopic.php?f=140&t=3754
- Tue Feb 09, 2016 8:22 pm
- Forum: Phase Changes & Related Calculations
- Topic: Winter 2013 Midterm Q2A.
- Replies: 4
- Views: 1104
Re: Winter 2013 Midterm Q2A.
The ice is melting and cooling down the water. Delta H fusion will be positive because it is the melting enthalpy (the name is kind of confusing, it seems like delta H fusion would be freezing but it is melting)
- Sat Feb 06, 2016 11:06 am
- Forum: Balancing Redox Reactions
- Topic: Winter 2011 Midterm Q#6
- Replies: 1
- Views: 395
Winter 2011 Midterm Q#6
In this question we were asked to balance a redox reaction and got:
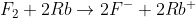
In the next step, we needed to calculate for Q, and 2Rb was excluded because it was a solid, but no states were given. Do we know how to assign states to the elements of a redox reaction?
In the next step, we needed to calculate for Q, and 2Rb was excluded because it was a solid, but no states were given. Do we know how to assign states to the elements of a redox reaction?
- Fri Feb 05, 2016 12:11 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3908957
Re: Chemistry Jokes
The name's Bond
... Ionic Bond
"taken, not shared"
... Ionic Bond
"taken, not shared"
- Sun Jan 31, 2016 3:30 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Self Test 11.5A Standard Gibbs
- Replies: 1
- Views: 432
Self Test 11.5A Standard Gibbs
"Use the thermodynamic data in Appendix 2A to calculate K from the value of  for the reaction
for the reaction
N204(g) -> 2NO2(g) at 298K."
Which element of the reaction do I look up for?
for?
Is it delta G formation of NO2? If so, do I multiply that by 2?
N204(g) -> 2NO2(g) at 298K."
Which element of the reaction do I look up
Is it delta G formation of NO2? If so, do I multiply that by 2?
- Fri Jan 22, 2016 11:33 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Homework Problem 9.19
- Replies: 1
- Views: 569
Homework Problem 9.19
9.19: "Calculate the standard entropy of vaporization of water at 85 degrees C, given that its standard entropy of vaporization at 100 degrees C is 109.0 J/K.mol and the molar heat capacities at constant pressure of liquid water and water vapor are 75.3 J/k.mol and 33.6 J/K.mol respectively in ...
- Mon Jan 18, 2016 12:01 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Constant Volume/Pressure Calorimeter
- Replies: 1
- Views: 1284
Constant Volume/Pressure Calorimeter
What is the difference between a constant volume and a constant pressure calorimeter?
Are there different equations to solve for each?
Are there different equations to solve for each?
- Fri Jan 15, 2016 7:10 pm
- Forum: Calculating Work of Expansion
- Topic: HW Problem 8.93 pressure of expansion
- Replies: 2
- Views: 1017
HW Problem 8.93 pressure of expansion
8.93 part a states: "Calculate the work that must be done against atmosphere for the expansion of the gaseous products in the combustion of 1.00 mol C6H6 (l) at 25 degrees celsius and 1 bar" I understand that you solve for the moles of gas, and rearrange the work expansion equation as foll...
- Sun Jan 10, 2016 9:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question 8.57
- Replies: 1
- Views: 523
Re: Question 8.57
I used this method as well, but you seem to have accidentally flipped products and reactants and somehow got it right. I did the following (-1560kJ)-[(-1300)+2(-286)] = 312 kJ (this is supposed to be negative) Is this a correct approach? If so, why are my signs not coming out correctly. The answer k...
- Sat Jan 09, 2016 8:24 pm
- Forum: Phase Changes & Related Calculations
- Topic: Homework Question 8.3
- Replies: 3
- Views: 2529
Re: Homework Question 8.3
In regards to part B of this question: (b) Is the work positive or negative with respect to the air in the pump? Is it correct to assume that the work is positive inside the pump because the gas is compressing and excerpting a greater force on the container as a result? Or it the work positive becau...
- Thu Nov 26, 2015 8:35 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Hund's Rule, Pauli Exclusion and Aufbau's Principle
- Replies: 3
- Views: 1393
Re: Hund's Rule, Pauli Exclusion and Aufbau's Principle
Here is the video in .mov format. It describes electron configuration notation and includes definitions of Hund's Rule, Pauli exclusion, and the Aufbau Principle
- Mon Oct 26, 2015 11:47 pm
- Forum: Bond Lengths & Energies
- Topic: Unpaired electron bond dissociation
- Replies: 1
- Views: 609
Unpaired electron bond dissociation
I understand that a lone pair would weaken surrounding bonds, but how would an unpaired electron affect the dissociation energy?
- Sun Oct 25, 2015 4:15 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Quiz #2 content
- Replies: 1
- Views: 520
Quiz #2 content
If Quiz #2 only covers Ch.3 and Ch.4 up through hybridization. Does that mean we aren't responsible for knowing how to do questions 4.51 on onward relating to molecular orbital theory? Should we complete the problems on the self quizzes related to bond order? Ex. #5 in the fall 2015 quiz 2 prep.
- Tue Oct 13, 2015 11:15 am
- Forum: Photoelectric Effect
- Topic: Fall 2013 Quiz Preperation Problem 10
- Replies: 4
- Views: 987
Re: Fall 2013 Quiz Preperation Problem 10
Do we keep track of sigfigs throughout the problem? ex. for the energy calculation I got for 1.023928759x10^-18 would I round that to 1.02x10^-18 considering it is limited by the given value 194 nm? Or do I plug in the whole value and only round according to sigfigs at the end. Also, I understand ho...
- Sun Oct 11, 2015 10:56 pm
- Forum: Empirical & Molecular Formulas
- Topic: Self Quiz Question 9
- Replies: 1
- Views: 481
Re: Self Quiz Question 9
You are on the right track. 2.38 can be multiplied by a whole number (a bit higher than 3) to get a whole number (it won't be exact, but close enough, within .1).
- Sun Oct 11, 2015 2:51 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chemical Nomenclature (quizzes)
- Replies: 1
- Views: 511
Chemical Nomenclature (quizzes)
I have minimal background in chemical nomenclature. Should we have the nomenclature of compounds in section D of the textbook committed to memory? Or are there some key common names for compounds that we should know for the upcoming quiz?
- Sun Oct 11, 2015 1:49 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Practice Quiz for Quiz 1
- Replies: 3
- Views: 723
Re: Practice Quiz for Quiz 1
You turn your workbook in to your TA before you take the quiz.
- Sun Oct 04, 2015 6:49 pm
- Forum: DeBroglie Equation
- Topic: De Broglie wavelength: How small is too small?
- Replies: 1
- Views: 713
De Broglie wavelength: How small is too small?
At what point are wave lengths calculated by the De Broglie equation too small to be measured? I understand that you can't see the wavelike properties of the car used in the example. Is anything larger than an electron too small to be measured? Are we responsible for knowing where this cutoff is?
- Sat Sep 26, 2015 5:37 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Calculating the sulfide of a metal
- Replies: 4
- Views: 1054
Re: Calculating the sulfide of a metal [Calcium?]
I understand how to calculate for the mass of the unknown variable M, but when does the calcium become involved? The question asks for the mass of the sulfide, I thought that was S2-. The answer key is talking about the molar mass of calcium sulfide.

