Search found 56 matches
- Wed Mar 09, 2016 5:15 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question 1A on 2011 Final
- Replies: 2
- Views: 702
Re: Question 1A on 2011 Final
There might be an error. You're right; you should calculate the q needed to raise the temperature to 100oC, the energy needed to vaporize, and the energy released when its cooled back to 25oC. You would only do this when carrying out an entropy of vaporization calculation at a temperature other tha...
- Thu Mar 03, 2016 9:00 am
- Forum: *Cycloalkenes
- Topic: parent chain
- Replies: 3
- Views: 1120
Re: parent chain
To add, the "parent" may not always be a single chain. The parent can be a cycloalkane, cycloalkene, or cycloalkyne if the cyclic chain contains more carbons than the substitutents. For example, a ring with 6 carbons will be called the substituent if it is attached to a propyl. The propyl ...
- Sun Feb 28, 2016 2:03 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.67 Homework Activation Energies
- Replies: 8
- Views: 2392
Re: 15.67 Homework Activation Energies
To add, I find it more intuitive to set r, or R, to 8.314 J/K mol and keep x as 125 x 103 J/mol
Of course, the approach described above works too
Of course, the approach described above works too
- Sun Feb 28, 2016 1:03 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Units for a rate constant
- Replies: 1
- Views: 495
Units for a rate constant
In problem 15.63, the units for the rate constant are L/mol-s. The problem asks to solve for the rate constant at another temperature, given the activation energy.
However, the answer in the back of the book has the units mol/L-s
Why are the units different?
Thank you!
However, the answer in the back of the book has the units mol/L-s
Why are the units different?
Thank you!
- Sun Feb 28, 2016 12:54 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: HW 15.63
- Replies: 2
- Views: 684
Re: HW 15.63
One important thing:
Note that the problem gives the temperatures in Celsius, so you would have to convert them to Kelvin.
Note that the problem gives the temperatures in Celsius, so you would have to convert them to Kelvin.
- Wed Feb 24, 2016 10:31 pm
- Forum: Second Order Reactions
- Topic: ions and reaction orders
- Replies: 2
- Views: 697
Re: ions and reaction orders
Yes, anything that is not an intermediate contributes to the order. Intermediates do not show up in the overall reaction.
- Thu Feb 18, 2016 10:25 pm
- Forum: Second Order Reactions
- Topic: Half life of second order reactions
- Replies: 2
- Views: 701
Re: Half life of second order reactions
To add, the time it takes for the concentration to halve is also not co start in a zero order reaction.
- Thu Feb 18, 2016 10:23 pm
- Forum: Second Order Reactions
- Topic: Activation Energy
- Replies: 2
- Views: 580
Re: Activation Energy
Also, catalysts lower the activation energy so the reaction happens more easily.
- Thu Feb 18, 2016 10:22 pm
- Forum: Second Order Reactions
- Topic: Rate
- Replies: 1
- Views: 615
Re: Rate
The rate constant in a second order reaction would be
the slope of the
line of the inverse of the concentration of reactant plotted against time.
the slope of the
line of the inverse of the concentration of reactant plotted against time.
- Sat Feb 06, 2016 11:57 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: 14.55 How to determine cathode and anode?
- Replies: 1
- Views: 786
Re: 14.55 How to determine cathode and anode?
Ni is the cathode because it's reduction reaction has a more positive standard reduction potential than SO 4 2- We don't need to change the sign because it is already accounted for when we subtract the cathode potential from the anode potential. If we were to change +1.23V to -1.23V, then we would a...
- Sat Feb 06, 2016 11:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.119 (aeration of a solution)
- Replies: 1
- Views: 510
14.119 (aeration of a solution)
Does aeration of a solution mean that oxygen is actually reduced in redox?
I infer that apparently it does, since the solutions manual includes the reduction of water and oxygen gas.
Can someone please validate this?
I infer that apparently it does, since the solutions manual includes the reduction of water and oxygen gas.
Can someone please validate this?
- Sat Feb 06, 2016 11:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Calculating Ecell (course reader)
- Replies: 2
- Views: 611
Re: Calculating Ecell (course reader)
An anode reduction potential in itself is not negative. However, when we calculate the the difference potential of the cell, we subtract the potential of the cathode from the potential of the anode. If we need to balance equations ( in terms of charge), then we flip the sign of the reduction potenti...
- Sat Feb 06, 2016 10:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Chapter 14 IN-TEXT Example 14.4: Self-Test 14.5A
- Replies: 2
- Views: 749
Re: Chapter 14 IN-TEXT Example 14.4: Self-Test 14.5A
SubparChemist wrote:
The final answer multiplies both the reduction and oxidation reactions by two, just to eliminate the fraction in the .5H2(g)->H^+ + 1e-
The half-reaction would actually be H2(g) --> 2H+(aq) + 2e-
- Sat Feb 06, 2016 10:16 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing cell diagram: figuring out order
- Replies: 2
- Views: 744
Re: Writing cell diagram: figuring out order
OH- is placed to the left of the Oxygens because it represents the solution that is connected to the salt bridge.
- Sat Feb 06, 2016 10:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.35 Typo?
- Replies: 2
- Views: 822
Re: 14.35 Typo?
Actually, it is, in fact, an error. It should be e-, not 2e-.
Chem_Mod verified this.
https://lavelle.chem.ucla.edu/forum/viewtopic.php?f=140&t=11410
Chem_Mod verified this.
https://lavelle.chem.ucla.edu/forum/viewtopic.php?f=140&t=11410
- Sat Feb 06, 2016 10:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Disproportionaction in Aqueous Solution (14.33b)
- Replies: 1
- Views: 478
Re: Disproportionaction in Aqueous Solution (14.33b)
We calculate the standard Gibbs free energy by adding the standard Gibbs free energies of the
two half reactions,
Tl--> Tl3+ + 3e-
and
Tl+ + e- --> Tl
two half reactions,
Tl--> Tl3+ + 3e-
and
Tl+ + e- --> Tl
- Sat Feb 06, 2016 7:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: example 14.3 in textbook
- Replies: 1
- Views: 516
Re: example 14.3 in textbook
Apparently there are 2 electrons transfered per mol in a Daniell cell. Considering that the battery uses copper and zinc, it would make sense that 2 electrons are transfered, since the reduction half-reaction of Zinc involves the transfer of two electrons, while the half-reaction of copper could inv...
- Sat Feb 06, 2016 7:01 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing Cell Diagrams
- Replies: 1
- Views: 491
Re: Writing Cell Diagrams
Platinum is an inert conductor, which basically means it can be used to conduct electricity complete a circuit. The electrons can flow through it. We add inert conductors such as platinum to the design of a battery when there are no conducting electrodes that we can get from the redox equation. Some...
- Sat Feb 06, 2016 6:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram 14.17 (b)
- Replies: 1
- Views: 491
Re: Cell Diagram 14.17 (b)
Yes, you are right. Use inert conductors such as platinum if there are no conducting metals already in the electrode.
- Wed Feb 03, 2016 7:39 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing redox reactions using other molecules
- Replies: 1
- Views: 473
Re: Balancing redox reactions using other molecules
For the use of H20, you can use OH and H in problems involving acidic solutions.
- Sat Jan 30, 2016 2:41 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation of C2H2O4
- Replies: 1
- Views: 1760
Re: Oxidation of C2H2O4
The oxidation number of an atom is derived from the bonds it makes from other atoms. An atom's charge is influenced by differences in electronegativity. For example, every C-H bond will decrease the oxidation state by 1, and every carbon bond to a more electronegative element will increase the oxida...
- Sat Jan 30, 2016 2:34 am
- Forum: Balancing Redox Reactions
- Topic: Voltage changing while balancing equations?
- Replies: 2
- Views: 1623
Re: Voltage changing while balancing equations?
Reduction potential is an intensive property, so it does not depend on quantity.
- Fri Jan 29, 2016 3:11 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3686658
Re: Chemistry Jokes
Learning about equilibrium is easy...
...because nothing changes
...because nothing changes
- Mon Jan 25, 2016 11:35 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Calculating residual entropy
- Replies: 1
- Views: 856
Calculating residual entropy
How would one calculate residual entropy, or is residual entropy something that will always be given?
- Mon Jan 25, 2016 4:50 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: #8 in Quiz Prep Winter 2011
- Replies: 6
- Views: 1239
Re: #8 in Quiz Prep Winter 2011
Would it be 610x23, or is that for a different equation?
- Mon Jan 25, 2016 4:08 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Phase Change Enthalpy
- Replies: 1
- Views: 586
Re: Phase Change Enthalpy
By enthalpy do you mean the enthalpy of formation? The enthalpy of formation of an element in its standard state is zero, but the enthalpy of vaporization fo a substance is not zero.
- Mon Jan 25, 2016 4:05 pm
- Forum: Calculating Work of Expansion
- Topic: Quiz Winter 2014 #11 Finding Final Volume
- Replies: 1
- Views: 504
Re: Quiz Winter 2014 #11 Finding Final Volume
Work is positive in step 2 because upon returning to the original internal energy, some of the decrease in the internal energy done in step 1 is undone.
- Mon Jan 25, 2016 3:59 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.83 Homework Problem: using volume to find concentration
- Replies: 3
- Views: 3501
Re: 983 Homework Problem: using volume to find concentration
This reaction is taking place presumably at standard temperature and pressure, so the pressure is 1 atm and the temperature is 0 degrees Celsius. Assuming the gas has ideal behavior, we can use PV= nRT to find the moles of gas. The pressure is at 1 atm, and the problem provides the volume, You can u...
- Mon Jan 25, 2016 3:49 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Reverse vs Forward Reaction
- Replies: 1
- Views: 537
Re: Reverse vs Forward Reaction
When a substance is in the gas phase, it is more disordered. An increase in entropy favors gases, as with more entropy comes more disorder.
When a substance is below the boiling point, entropy wants to decrease.
When a substance is below the boiling point, entropy wants to decrease.
- Mon Jan 25, 2016 3:35 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Which R to use
- Replies: 1
- Views: 544
Re: Which R to use
Yes, the R's related to heat capacity and work are all equivalent. You choose which one to use based off of which units you need to accommodate.
- Mon Jan 25, 2016 3:07 pm
- Forum: Calculating Work of Expansion
- Topic: Isothermal expansion vs compression
- Replies: 1
- Views: 633
Isothermal expansion vs compression
Do we use the same equation, 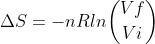 for both isothermal expansions and compressions? If so, what is the conceptual reasoning behind it?
for both isothermal expansions and compressions? If so, what is the conceptual reasoning behind it?
- Sun Jan 24, 2016 1:32 pm
- Forum: Environment, Fossil Fuels, Alternative Fuels
- Topic: Do alternate fuels create less entropy?
- Replies: 3
- Views: 1595
Re: Do alternate fuels create less entropy?
Are you referring to an increase in entropy of a particular system? An increase compared to current fuels (if so, which fuels)? An increase in entropy is an increase in the number of particles and/or kinetic energy. Biodiesel fuels have a high enthalpy density, while methane has a low enthalpy densi...
- Fri Jan 22, 2016 12:39 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Implications of Constant temperature, pressure and volume
- Replies: 1
- Views: 510
Re: Implications of Constant temperature, pressure and volum
Processes involving constant temperature are also known as isothermal processes. Isothermal processes can mean that heat is put into a system. However, there is no change in internal energy because all of the heat energy is used to do work. Recall the Pressure vs. Volume graph. To calculate the work...
- Fri Jan 22, 2016 12:21 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: #8 in Quiz Prep Winter 2011
- Replies: 6
- Views: 1239
Re: #8 in Quiz Prep Winter 2011
Is this in the purple workbook for Winter 2016?
- Fri Jan 22, 2016 12:16 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Actual Entropy vs. Boltzmann Entropy
- Replies: 1
- Views: 509
Re: Actual Entropy vs. Boltzmann Entropy
If the actual entropy is close to the second entropy (the experimental value), it helps us find out how molecules are arranged in a solid. The purpose of the Boltzmann formula is to show that the orientations a molecule can have is related to its entropy. It appears, from looking at example 9.8, tha...
- Fri Jan 22, 2016 11:42 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Definition
- Replies: 2
- Views: 697
Re: Gibbs Free Energy Definition
Free energy is energy available to do work.
- Fri Jan 22, 2016 9:36 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Rate of Entropy Generation
- Replies: 3
- Views: 850
Re: Rate of Entropy Generation
Keep in mind, heat transfer can change entropy. If heat leaves a system, the entropy decreases. Considering that the entropy of the universe is zero, somewhere else there has to be an entropy change that is positive to balance out the first entropy change.
- Fri Jan 22, 2016 9:32 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Rate of Entropy Generation
- Replies: 3
- Views: 850
Re: Rate of Entropy Generation
Entropy, being a measure of disorder, can change when the reactant undergoes a phase change. If the reactant changes from a gas to a liquid, the entropy decreases, because a substance is more disordered as a gas than as its liquid.
- Fri Jan 22, 2016 9:23 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Internal Energy and Temperature
- Replies: 1
- Views: 389
Re: Internal Energy and Temperature
If you're measuring change in internal energy based off of q when heating a system under constant volume, you would use q=nCvdelta T
Here, if delta T is zero, q is zero and the change in internal energy is zero.
Here, if delta T is zero, q is zero and the change in internal energy is zero.
- Sun Jan 17, 2016 6:35 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: different variables
- Replies: 1
- Views: 450
Re: different variables
Here's a hypothetical example: A system, defined as a metal pot, is heated with a stove that has an output of 100 joules per second. The pot is heated for 5 seconds. While the pot is heated, it turns out that the pot (the system ) underwent a change in internal energy of +1000 joules. Calculate the ...
- Sun Jan 10, 2016 4:20 pm
- Forum: Phase Changes & Related Calculations
- Topic: change in enthalpy value
- Replies: 3
- Views: 739
Re: change in enthalpy value
But how do you know what the sign of the forward reaction was to begin with? is that dependent on whether the reaction is endothermic or exothermic? Yes, an enthalpy value for a reaction depends on whether it's endo- or exo- thermic. For example, consider the reaction of methane with water vapor. C...
- Sat Dec 05, 2015 6:42 pm
- Forum: *Titrations & Titration Calculations
- Topic: Half-ing molarities?
- Replies: 1
- Views: 679
Half-ing molarities?
In question 13.35, part f) asks to find the pH at the stoichiometric point. In this exercise, CH 3 COOH is the weak acid with a molarity of 0.1. It's titrated with 0.1 M NaOH. Here the solutions manual, when solving for pH at the equilibrium point, sets the molarity of CH 3 CO 2 - as 0.05 M. Why is ...
- Sat Dec 05, 2015 5:39 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Quantum Numbers
- Replies: 3
- Views: 802
Re: Quantum Numbers
What is the maximum number of electrons in an atom that can these quantum numbers? n=2 m1=1 ms=+1/2 Let's consider that energy level (N) equals 2. Therefore, L can equal 0 and 1. L=0 represents the s-orbital. L=1 represents the p-orbital. For the s-orbital, an electron can have 1 orientation, since...
- Sat Dec 05, 2015 5:23 pm
- Forum: Conjugate Acids & Bases
- Topic: Equilibrium sign or not [ENDORSED]
- Replies: 4
- Views: 1182
Re: Equilibrium sign or not [ENDORSED]
The dissociation of HCL is not a reversible reaction. Therefore, an equilibrium sign may not be appropriate. In theory, an equilibrium sign would be appropriate only if: You have a flask containing HCL and water. HCL was dissociating into H+ and Cl- at a certain rate. Somehow, H+ and Cl- were formin...
- Sat Dec 05, 2015 5:17 pm
- Forum: Lewis Structures
- Topic: 2011 Final Q5B: Lewis Structure
- Replies: 2
- Views: 719
Re: 2011 Final Q5B: Lewis Structure
Additionally, the Cl would not be able to fit in, as the S would have a full octet.
But wait...S can break the octet rule...
But wait...S can break the octet rule...
- Sat Dec 05, 2015 5:14 pm
- Forum: *Titrations & Titration Calculations
- Topic: 12.23
- Replies: 11
- Views: 2671
Re: 12.23
But how exactly do we find the final volume ( which is after the stoichiometric point)? The stoichiometric point is when the pH is 7 and the total volume is 10ml, but this is not the final point. Theoretically, more HCL would be added so that the [HCL] = [NaOH], but is there a way to find how much e...
- Sat Dec 05, 2015 4:50 pm
- Forum: *Titrations & Titration Calculations
- Topic: 12.23
- Replies: 11
- Views: 2671
Re: 12.23
Would we be required to find the final volume? In this case, is finding the final volume even possible?
- Sat Nov 28, 2015 11:42 pm
- Forum: Hybridization
- Topic: VIDEO: Hybridization and bonding
- Replies: 1
- Views: 607
VIDEO: Hybridization and bonding
Alexander Vong
504598309
Discussion 1D
I hope this is helpful!
504598309
Discussion 1D
I hope this is helpful!
- Tue Nov 17, 2015 9:55 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxygen coordination
- Replies: 1
- Views: 578
Oxygen coordination
Say a ligand were to consist of a cation and multiple oxygens. If there were oxygens with 2 lone pairs but also oxygens atoms with 3 lone pairs, which oxygen atom would be used for determining the number of coordinate bonds made?
- Fri Nov 13, 2015 10:04 pm
- Forum: Hybridization
- Topic: Writing sp3d2 in the correct order
- Replies: 1
- Views: 627
Writing sp3d2 in the correct order
Say an atom is surrounded by 6 areas of electron density, and therefore the hybridization is sp3d2. Would the d2 part be written before sp3 or after?
- Tue Oct 27, 2015 10:17 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: The meaning of "main group metal"
- Replies: 1
- Views: 620
The meaning of "main group metal"
The term "main group metal" appears on p. 66 of the course reader. To quote: " Main group metal forms cation: loses s and p valence e- (e- configuration of preceding inert-gas atom). p-block element forms anion: gains e- until following noble-gas e- configuration is reached " Do ...
- Tue Oct 27, 2015 9:57 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Course reader clarification: -VE
- Replies: 1
- Views: 582
Course reader clarification: -VE
The abbreviation, "-VE", appears in the course reader several times. Could someone please clarify the meaning of VE? It appears in the following context: (on p.75) An e- pair in a covalent bond may not be equally shared [between two atoms] The atom with the closer e- pair is slightly -VE( ...
- Fri Oct 23, 2015 3:11 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Hybridization: Orbitals and shells
- Replies: 1
- Views: 399
Hybridization: Orbitals and shells
When n=2, and a s orbital and 2 p orbitals are hybridized, is 2sp2 a shell or an orbital?
- Fri Oct 16, 2015 11:23 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Work function equation sign
- Replies: 1
- Views: 521
Work function equation sign
In the work function equation, can Energy emitted be negative?
- Thu Oct 08, 2015 10:57 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Clarification on the use of noble gases in configurations
- Replies: 2
- Views: 664
Clarification on the use of noble gases in configurations
Is it more important on say, a quiz, to know how to write an entire electron configuration without using noble gases to denote non-valence electrons, or are we allowed to write just the noble gas core and then the valence electrons? For example, consider calcium. Would it be important to know how to...
- Thu Oct 01, 2015 9:23 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Clarification on modeling an atom
- Replies: 1
- Views: 549
Clarification on modeling an atom
Exercise 1.43 from Chapter 1 asks, "What is the minimum uncertainty in the speed of an electron confined to within a lead atom of diameter 350. pm? Model the atom as a one-dimensional box with a length equal to the diameter of the actual atom." Could someone please clarify what "model...

