Search found 21 matches
- Sat Feb 11, 2017 4:28 pm
- Forum: Balancing Redox Reactions
- Topic: Ozone as an oxidizing agent [ENDORSED]
- Replies: 1
- Views: 516
Ozone as an oxidizing agent [ENDORSED]
This question is in reference to 14.5 the equation: O_3(aq)+Br^-(aq) \rightarrow O_2(g)+BrO_3^-(aq) O_3 is the oxidizing agent, but I'm having trouble seeing why because both O_3 and O_2 are neutral. Can somebody please explain to me why O_3 is the oxidizing agent? Is...
- Mon Jan 23, 2017 9:53 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Molar Heat Capacities
- Replies: 1
- Views: 511
Re: Molar Heat Capacities
You can find all the given formulas for the exams on page 125 of your course reader, titled: Constants and Formulas
- Mon Jan 23, 2017 9:39 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Calculating the change in entropy of the surroundings
- Replies: 1
- Views: 518
Calculating the change in entropy of the surroundings
Where does the equation 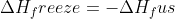 come from? This is used in example 9.10 when calculating the change in entry of the surroundings when mercury freezes at 224K and
come from? This is used in example 9.10 when calculating the change in entry of the surroundings when mercury freezes at 224K and 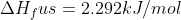
- Sat Mar 12, 2016 9:35 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: reaction enthalpy
- Replies: 1
- Views: 409
reaction enthalpy
Would reaction enthalpy (deltaH) multiply by 2 if you multiplied the entire equation by 2?
Would Gibbs do this?
Would Gibbs do this?
- Tue Mar 08, 2016 9:49 pm
- Forum: Balancing Redox Reactions
- Topic: Making a battery
- Replies: 1
- Views: 472
Making a battery
When designing a battery and given two values when given Estandard values Ex. F2 (g) + 2H^+(aq) + 2 e^- -----> 2HF (aq) E standard=+3.03 V F2 (g) +2e^- -------> 2F^-(aq) E standard=+2.87 V Do we reverse any of the reactions to get negative E standard values? Or do we plug them in just the way they a...
- Wed Mar 02, 2016 8:50 pm
- Forum: *Nucleophilic Substitution
- Topic: Transition States
- Replies: 2
- Views: 1676
Re: Transition States
Very helpful, thanks!
- Sun Feb 28, 2016 4:29 pm
- Forum: *Nucleophilic Substitution
- Topic: Transition States
- Replies: 2
- Views: 1676
Transition States
I don't understand where the SN1 and SN2 type of reactions are coming from. Where are they coming from? What do they apply to? Are they in the course reader? Are they talked about in the intro to OChem book? Please help!
- Sat Feb 27, 2016 10:34 pm
- Forum: *Alkanes
- Topic: Parent chain -ENE numbering
- Replies: 1
- Views: 246
Parent chain -ENE numbering
I understand that parent chains are named last. However, I'm not sure if parent chains are supposed to receive the lowest numbering. For example, in Quiz 3 Prep1 Question 3, it asks for the name of CH3CH=C(CH3)CH(CH3)2 the answer gives 3,4-dimethyl-2-pentene.
I would have put 2,3-dimethyl-3-pentene.
I would have put 2,3-dimethyl-3-pentene.
- Sun Feb 21, 2016 3:58 pm
- Forum: *Electrophiles
- Topic: Positively charged Carbon
- Replies: 1
- Views: 847
Positively charged Carbon
Why is it that, in the introduction to OChem textbook, H3C--C^+H--CH3 has a middle carbon that is positively charged, but has six valence electrons? I thought that since Carbon typically has four valence electrons, six valence electrons would make it into a negative charge? Someone please explain. T...
- Tue Jan 26, 2016 8:19 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Adding equations to get a final equation (deltaH)
- Replies: 2
- Views: 607
Re: Adding equations to get a final equation (deltaH)
Yes, thank you so much!!
- Tue Jan 26, 2016 7:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Adding equations to get a final equation (deltaH)
- Replies: 2
- Views: 607
Adding equations to get a final equation (deltaH)
So the first practice quiz, question 4, you are trying to get the final equation: 2A+D-->C using the equations 1) 2A+B-->3C+E 2) 2B-->A+D+E 3) E-->3B+C The workbook says the correct way to do it is by taking 1/2DH1-DH2-1/2DH3 (Where D is delta). I tried that, but I'm not getting the final equation. ...
- Sun Jan 24, 2016 5:10 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Compression
- Replies: 2
- Views: 679
Re: Compression
Super helpful, thanks (:
- Tue Jan 19, 2016 11:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Compression
- Replies: 2
- Views: 679
Compression
I'm having trouble keeping the work of compression straight.
When gas in a system is compressed is the work done ON or BY the system?
Also, why is it that w and u are positive when gas in a system is compressed? Is it because the smaller volume encourages collisions?
When gas in a system is compressed is the work done ON or BY the system?
Also, why is it that w and u are positive when gas in a system is compressed? Is it because the smaller volume encourages collisions?
- Mon Nov 02, 2015 10:13 am
- Forum: Hybridization
- Topic: Lone Pair/Unpaired Electron
- Replies: 2
- Views: 2288
Re: Lone Pair/Unpaired Electron
You mostly have to have your VSPER shapes memorized, as you need to be able to recognize that something with four bonds is tetrahedral, whereas something with four bonds and two lone pairs is square planar (due to electron repulsion). For hybridization you can count the regions of electron density. ...
- Sun Oct 25, 2015 8:18 pm
- Forum: Octet Exceptions
- Topic: explanation for ClO2 lewis structure (problem 67b)
- Replies: 1
- Views: 1142
Re: explanation for ClO2 lewis structure (problem 67b)
The question is asking why the lewis structure is wrong. So here, it is not allowed to violate the octet rule the way it does because it has an odd number of electrons around the central atom. Cl can, however violate the octet rule, by having an expanded octet, but that isn't the case here.
- Sun Oct 25, 2015 8:14 pm
- Forum: Lewis Structures
- Topic: Formal charge
- Replies: 1
- Views: 371
Re: Formal charge
Yes. So, for example, if you have something like [ICl_2]+ , you should have two neutral chlorines singly bonded to an Iodine that is missing 1 electron, which makes the overall charge positive.
- Sat Oct 24, 2015 8:19 pm
- Forum: Electronegativity
- Topic: Ionic character and electronegativity
- Replies: 1
- Views: 557
Ionic character and electronegativity
Why is it that HCl is more ionic in character than HI ?
I would think that the electronegativity difference between H and I is greater than H and Cl, which would make HI more ionic in character than HCl.
I would think that the electronegativity difference between H and I is greater than H and Cl, which would make HI more ionic in character than HCl.
- Sat Oct 24, 2015 7:09 pm
- Forum: Lewis Structures
- Topic: Lewis Structure XeF_2
- Replies: 4
- Views: 2311
Lewis Structure XeF_2
Is there a reason why the lewis structure for XeF_2 is drawn vertically rather than horizontally?
- Sun Oct 11, 2015 9:48 am
- Forum: Empirical & Molecular Formulas
- Topic: Empirical formula with Co and F
- Replies: 1
- Views: 911
Empirical formula with Co and F
So: 339.20g Co metal is reacted with compressed F gas to produce a compound with a mass of 996.08g. What is the empirical formula of the new compound? (Atomic Masses: Co (58.93g/mol) F (19.00g/mol) ). I have absolutely no idea what to do first/ how to find the masses that are needed to convert to mo...
- Sun Oct 11, 2015 9:38 am
- Forum: Trends in The Periodic Table
- Topic: Electron Affininty
- Replies: 1
- Views: 486
Re: Electron Affininty
So, as you move down a group, the shielding effect increases which likewise increases the repulsion between electrons. Going down a group also places electrons in higher energy levels that are further away from the nucleus. With these two factors in mind, the effective nuclear charge is much lower o...
- Tue Sep 29, 2015 7:17 pm
- Forum: Properties of Light
- Topic: HW 1.7A
- Replies: 3
- Views: 1271
HW 1.7A
The question in the book asks: The frequency of violet light is 7.1x10^14Hz. What is the wavelength (in nanometers) of the violet light? So here's what I did: I used the equation λ = c / ν to find wavelength in meters which was 4.2x10^-7 . I'm having trouble converting to nanometers. Where the solut...

