Search found 14 matches
- Tue Mar 08, 2016 4:46 pm
- Forum: *Alkanes and Substituted Alkanes (Staggered, Eclipsed, Gauche, Anti, Newman Projections)
- Topic: Torsional strain
- Replies: 4
- Views: 1060
Torsional strain
Would torsional strain be negative if you were moving from a less stable conformation to a more stable one?
- Tue Mar 01, 2016 1:03 am
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Preference in numbering
- Replies: 10
- Views: 5080
Re: Preference in numbering
Do halides have priority over double bonds? I'm referring specifically to 1,6-dichloro-cyclohexane on page 105 of the course reader.
- Mon Feb 29, 2016 11:03 pm
- Forum: *Haloalkanes
- Topic: Placement of halide substituent in IUPAC name
- Replies: 1
- Views: 1224
Placement of halide substituent in IUPAC name
Do halides always come first in the name or does it go alphabetically? (I.e. If there was an ethyl and a fluorine anion attached to the same carbon, which would be listed first?)
- Mon Feb 29, 2016 11:00 pm
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Preference in numbering
- Replies: 10
- Views: 5080
Preference in numbering
I know you give preference to double bonds and functional groups when numbering, but which is more important? If you had a choice between setting a functional group at the second carbon or a double bond starting at the second carbon, which would you choose?
- Sat Feb 27, 2016 4:02 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Possible error on page 163 of ochem textbook
- Replies: 1
- Views: 487
Possible error on page 163 of ochem textbook
On page 163 of the Intro to Organic Chemistry textbook, it says "When \Delta G^o r <0, the exergonic reaction is favorable and K>1. When \Delta G^o r >0, the endergonic reaction is unfavorable and K<1." I believe there's an error here, but I just wanted to make sure. Im also unsure about w...
- Sun Feb 21, 2016 7:03 pm
- Forum: *Nucleophiles
- Topic: Is it possible to have two nucleotides in a rxn?
- Replies: 1
- Views: 467
Is it possible to have two nucleotides in a rxn?
In example 2 on course reader page 85, it is stated that a nucleophile (denoted :Nu) donates electrons to and bonds with the central carbon atom. The Cl connected to the carbon is also electron rich because of its higher electronegativity. Is it considered a nucleoniphile as well or do nucleophiles ...
- Sun Feb 14, 2016 3:55 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Equation to find k when there's more than 1 reactant
- Replies: 2
- Views: 564
Equation to find k when there's more than 1 reactant
On page 69 of the course reader, it says 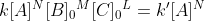 . Why is A not an initial concentration?
. Why is A not an initial concentration?
- Sun Feb 14, 2016 3:48 pm
- Forum: Second Order Reactions
- Topic: Converting 2nd order diff rate law to integrated rate law
- Replies: 1
- Views: 533
Converting 2nd order diff rate law to integrated rate law
I'm confused as to where the 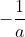 goes when you integrate the 2nd order differential rate law,
goes when you integrate the 2nd order differential rate law, 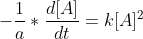 , to get
, to get 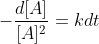 . Are we assuming a equals 1? If so, why?
. Are we assuming a equals 1? If so, why?
- Sat Feb 06, 2016 1:40 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Max potential
- Replies: 1
- Views: 440
Max potential
It is stated that potential is maximized when the process is reversible and there's very little current, but what is the explanation behind this?
- Sat Feb 06, 2016 1:36 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Definition of cell and redox potential
- Replies: 1
- Views: 598
Definition of cell and redox potential
What is the difference between E0redox and E0cell? On page 50 of the course reader, when we are given two half reactions, we want to reverse the sign of the iron half reaction to get a positive cell potential. In the gold example on page 52, E0redox is allowed to be negative. Why is this?
- Sun Jan 31, 2016 11:11 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Definition of microstate
- Replies: 1
- Views: 1676
Definition of microstate
What exactly is a microstate? Is it a location, an energy level, or something else?
- Sat Jan 23, 2016 12:01 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Difference between q and delta h
- Replies: 1
- Views: 1056
Difference between q and delta h
If enthalpy is the amount of heat released and absorbed at a constant pressure, why does the course reader and textbook alternate between using q and 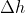 ? Is
? Is 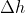 used when pressure isn't constant?
used when pressure isn't constant?
- Sun Jan 17, 2016 1:01 am
- Forum: Calculating Work of Expansion
- Topic: Work and equilibrium
- Replies: 1
- Views: 511
Work and equilibrium
What did Prof. Lavelle mean when he said "the most efficient work is done along an equilibrium pathway"?
- Sun Jan 10, 2016 11:39 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: State functions
- Replies: 4
- Views: 823
State functions
What are some other examples of state functions?

