Search found 13 matches
- Fri Mar 11, 2016 1:12 am
- Forum: *Organic Reaction Mechanisms in General
- Topic: Electron Transfers
- Replies: 1
- Views: 440
Electron Transfers
Are there any tips for drawing in electron transfers between two organic compounds, for instance, in an SN2 reaction?
- Fri Mar 04, 2016 1:12 am
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Z, E vs. cis, trans
- Replies: 1
- Views: 541
Z, E vs. cis, trans
How do you differentiate Z and E vs. cis and trans notation?
- Fri Feb 26, 2016 12:27 pm
- Forum: *Cycloalkenes
- Topic: Numbering for Cycloalkenes
- Replies: 2
- Views: 1045
Numbering for Cycloalkenes
How does the numbering for cycloalkenes work? Do you have the lowest number next to the double bonds or largest substituent?
- Thu Feb 18, 2016 10:33 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Determining which order a reaction is
- Replies: 1
- Views: 483
Re: Determining which order a reaction is
You can also look at the sum of the exponents in the rate law. The rate is given by: Rate=k[A]^n[B]^m[C]^l... and the order will be n+m+l+... . You can also look at what is displayed on a graph. If it is ln(A_{t}) vs. time, you can infer that this is a first order equation. If a graph has \f...
- Thu Feb 11, 2016 11:57 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Elementary Reactions
- Replies: 1
- Views: 433
Elementary Reactions
Do transition states appear in elementary reactions at all?
- Wed Feb 03, 2016 1:01 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Problem 14.21c
- Replies: 3
- Views: 654
Re: Problem 14.21c
Is the Cl- ion in the cell diagram shown as the product of the oxidation half reaction then? Why is it included?
- Tue Feb 02, 2016 11:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Problem 14.21c
- Replies: 3
- Views: 654
Problem 14.21c
In problem 14.21c, the problem provides the following cell diagram for us to find the cell potential: Hg_{(l)}|Hg_{2}Cl_{2}_{(s)}|Cl^{-}_{(aq)}||Hg^{2+}_{(aq)}|Hg_{(l)} . How would you perform the oxidation at the anode provided that Hg_{2}Cl_{2}_{(s)}...
- Thu Jan 28, 2016 9:24 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Physical Significance of Gibbs Free Energy
- Replies: 1
- Views: 5636
Physical Significance of Gibbs Free Energy
Gibbs free energy is mathematically defined as: 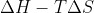 ; what does this value physically represent in the ongoing reaction?
; what does this value physically represent in the ongoing reaction?
- Sat Jan 23, 2016 10:43 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Finding molar entropy of benzene
- Replies: 1
- Views: 578
Re: Finding molar entropy of benzene
It doesn't seem like it would work this way. For instance, in the following link: www2.stetson.edu/~wgrubbs/datadriven/entropyaluminumoxide/entropyal2o3wtg.html, the molar entropy of Aluminum Oxide is found by integrating with respect to temperature the equation: \int_{0}^{T}\frac{C_{P}}{T}dT , wher...
- Sat Jan 23, 2016 8:43 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Negative Reaction Entropies for Ions
- Replies: 1
- Views: 528
Negative Reaction Entropies for Ions
Why do ions in solution sometimes have negative entropies? How do they make a system more ordered?
- Tue Jan 19, 2016 10:29 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Calculating W
- Replies: 1
- Views: 318
Calculating W
For calculating W given a particular molecule, do you just determine it based off the number of orientations that the molecule can occupy? For instance, for H2, can we state it only has one orientation and microstate?
- Wed Jan 13, 2016 1:23 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Change in Internal Energy with Constant Volume
- Replies: 1
- Views: 428
Change in Internal Energy with Constant Volume
When there is a constant volume, but non constant pressure, how do we calculate change in internal energy? Is it just the heat exchanged between the system and its surroundings?
- Wed Jan 13, 2016 1:16 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3696465
Re: Chemistry Jokes
Why is an electron always depressed?
It's quant-numb and uncertain of its place in the world.
It's quant-numb and uncertain of its place in the world.

