Search found 16 matches
- Wed Mar 09, 2016 2:25 pm
- Forum: Phase Changes & Related Calculations
- Topic: 2010 Final Q1A
- Replies: 1
- Views: 387
2010 Final Q1A
When solving for q water = -nC p,water ΔT, why is ΔT solved by doing (T f - 20 o C), when we should convert the 20 o C to 293K in order to cancel out units of the molar heat capacity of water (75.5 J/Kmol)?? I tried doing the calculation with 293K, in order to properly cancel out the units, and I ge...
- Tue Mar 08, 2016 7:30 pm
- Forum: *Calculations Using ΔG° = -RT ln K
- Topic: Confirmation Energy Differences
- Replies: 1
- Views: 1167
Confirmation Energy Differences
When using the calculation ΔG = -RTlnK will we be given the energy values for the different confirmations. For example, when solving for Gauche Butane ↔ Anti Butane, will we be told that Gauche Butane is 0.9 kcal/mol and anti butane is 0? Or should we have some values memorized?
- Wed Mar 02, 2016 5:58 pm
- Forum: *Cycloalkanes
- Topic: EAS vs. Electrophilic Addition Reaction
- Replies: 1
- Views: 542
EAS vs. Electrophilic Addition Reaction
For a reaction to be considered an electrophilic aromatic substitution (EAS), does it have to involve a benzene ring? Or would the reaction without a benzene ring (but still a cycloalkene) be considered an electrophilic addition reaction? Basically I am wondering if the two reaction terms are the sa...
- Wed Mar 02, 2016 4:11 pm
- Forum: *Cycloalkanes
- Topic: Naming Structures
- Replies: 2
- Views: 592
Re: Naming Structures
The answer would be 2-isopropyl-1,1-dimethylpentane because it is better to have two 1's rather than two 2's (we want the lowest possible numbers in the answer). Alphabetical order does not determine the numbering and the first substituent does not need to have the lowest number.
- Sat Feb 27, 2016 9:14 pm
- Forum: *Nucleophilic Substitution
- Topic: Drawing Reaction Mechanisms & Arrows
- Replies: 1
- Views: 515
Drawing Reaction Mechanisms & Arrows
When drawing the electron-flow arrows for reaction mechanisms, do we always have to indicate the dipole of the molecule(s) (by writing either δ - or δ + ) to explain why we are drawing the arrows if the question just states to "complete the reaction mechanism" or "used curved arrows t...
- Tue Feb 16, 2016 10:18 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Homework 15.23 part (b)
- Replies: 1
- Views: 455
Homework 15.23 part (b)
"Determine the rate constant for each of the following first-order reactions, in each case ex[ressed for the rate loss of A: ... (b) A->B, given that the concentration of A decreases from 0.67 mol/L to 0.53 mol/L in 25 s" Why would you use ln\left ( \frac{[A]_{o}}{[A]_{t}} \right )...
- Mon Feb 08, 2016 9:11 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Winter 2014 Midterm 1B
- Replies: 1
- Views: 439
Winter 2014 Midterm 1B
"Suppose 64.0 gram each of methyl alcohol, CH 3 OH (MW 32.0 g/mol) and ammonia, NH 3 (MW 17.0 g/mol) are allowed to react according to the above equation at 298 K and constant pressure. What is the heat q of the reaction?" I understand that we must first calculate the moles of the reaction...
- Sat Feb 06, 2016 11:03 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Winter 2012 Midterm Question 7B
- Replies: 1
- Views: 510
Winter 2012 Midterm Question 7B
"Q7B. Determine the equilibrium constant for the reaction: Mn (s) + Ti +2 (aq) --> Mn 2+ (aq) + Ti (s) " After balancing the half reactions, I got: Mn (s) -> Mn 2+ (aq) + 2e - 2e - + Ti +2 (aq) --> Ti (s) When using lnK = \fra...
- Tue Feb 02, 2016 9:15 pm
- Forum: Balancing Redox Reactions
- Topic: Half-Reactions
- Replies: 3
- Views: 758
Half-Reactions
When askedd to balance skeletal equations by using oxidation and reduction half-reactions, these half-reactions will be given to us? Because in some of the homework questions I could not find certain half-reactions. For example, the following half-reaction is not in Appendex 2, A 18 C 2 H 5 OH (...
- Mon Feb 01, 2016 2:18 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3681854
Re: Chemistry Jokes
I thought this was a cute image :) 

- Mon Jan 25, 2016 9:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Homework Question 8.25
- Replies: 3
- Views: 954
Re: Homework Question 8.25
Because a change in temperature, whether in Kelvin or in Celsius, will still result in the same difference (since changing to Kelvin you just add 273 to both temperature values, it will make no difference in your answer).
- Mon Jan 25, 2016 9:12 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Homework 9.45
- Replies: 2
- Views: 1632
Homework 9.45
"9.45 Use the information in Table 8.3 to calculate the changes in entropy f the surroundings and of the system for (a) the vaporization of 1.00 mol CH 4 (l) at its normal boiling point; (b) the melting of 1.00 mol C 2 H 5 OH(s) at its normal melting point; (c) the freezing of 1.00 mol C 2 H 5 ...
- Thu Jan 21, 2016 8:54 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: value of n in w= -nRT*ln(V2/V1) vs. q=nCΔT
- Replies: 2
- Views: 1598
value of n in w= -nRT*ln(V2/V1) vs. q=nCΔT
In some homework questions, the value of n in q = nCΔT is assumed to be equal to 1; however, when using w = 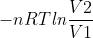 you must solve for n by PV = nRT.
you must solve for n by PV = nRT.
Why is this and is this always the case?
Why is this and is this always the case?
- Thu Jan 21, 2016 8:34 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Homework Question 8.25
- Replies: 3
- Views: 954
Homework Question 8.25
"8.25 A constant-volume calorimeter was calibrated by carrying out a reaction known to release 3.50 kJ of heat in 0.200 L of solution in the calorimeter (q = -3.50 kJ), resulting in a temperature rise of 7.23 o C. In a subsequent experiment, 100.0 mL of 0.200 M HBr (aq) and 100.0 mL of 0.200 M ...
- Thu Jan 21, 2016 6:19 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Homework Question 8.3 part (c)
- Replies: 1
- Views: 654
Homework Question 8.3 part (c)
"8.3 Air in a bicycle pump is compressed by pushing in the handle. If the inner diameter of the pump is 3.0 cm and the pump is depressed 20. cm with a pressure of 2.00 atm, (a) how much work is done in the compression? (b) is the work positive or negative with respect to the air in the pump (c)...
- Sun Jan 10, 2016 2:27 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HW 8.65
- Replies: 1
- Views: 462
HW 8.65
HW question 8.65 asks "Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data, 2NO(g) + O 2 (g) -> 2NO 2 (g) ∆H o = -114.1 kJ 4NO 2 (g) + O 2 (g) -> 2N 2 O 5 (g) ∆H o = -110.2 kJ and from the standard enthalpy of form...

