Search found 71 matches
- Sun Dec 06, 2015 12:23 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: C2+ or C2- bond strength
- Replies: 3
- Views: 11869
Re: C2+ or C2- bond strength
Yes, I checked again and realized I put in the wrong number of electrons. Thank you both for your help!
- Fri Dec 04, 2015 10:39 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Fall 2010 Final Exam
- Replies: 1
- Views: 642
Re: Fall 2010 Final Exam
It looks like you have the previous steps correct (making ΔE or hv negative because light was emitted, using the right constants, etc.) and all you need to do is solve for n. Multiply both sides by n 2 , so you will have 1 = (n 2 )(2.78x10 -2 ). Then divide both sides by 2.78x10 -2 to get 1/(2.78x10...
- Fri Dec 04, 2015 8:56 pm
- Forum: Properties of Electrons
- Topic: Number of electrons in a subshell [ENDORSED]
- Replies: 1
- Views: 919
Re: Number of electrons in a subshell [ENDORSED]
In other words, the question is asking for the maximum number of electrons that can be in the orbitals determined. (They would have the same quantum numbers, besides the magnetic spin, if located in the same orbitals). Because the orbitals are d orbitals, there are 5 of them in total and each can ho...
- Fri Dec 04, 2015 7:24 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: C2+ or C2- bond strength
- Replies: 3
- Views: 11869
C2+ or C2- bond strength
Question 4, part A of the 2010 final asks whether C 2 + or C 2 - has the stronger bond, and I determined C 2 + to be stronger since it has the higher bond order. But the answer says that C 2 - is stronger since the extra electron is in a bonding molecular orbital. Is this true? When I drew the MO di...
- Wed Dec 02, 2015 6:14 pm
- Forum: Conjugate Acids & Bases
- Topic: Percentage Deprotonated
- Replies: 3
- Views: 987
Re: Percentage Deprotonated
You would need to use [A-] for the deprotonation equation in that case, because the definition of deprotonation is the conversion of the acid HA to its conjugate base A-.
- Wed Dec 02, 2015 6:13 pm
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: Question about Buffers and Use of a Salt
- Replies: 4
- Views: 1052
Re: Question about Buffers and Use of a Salt
Yes, the conjugate base of a weak acid would be stronger than that of a strong acid, though it wouldn't be as strong a base as the ones listed in the course reader. In a buffer system it is optimal to have [HA]=[A-] to keep the pH constant, because if the amounts were different the addition of an ac...
- Wed Dec 02, 2015 6:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Video: Quiz 3 Problem 8
- Replies: 3
- Views: 747
Video: Quiz 3 Problem 8
This video involves calculating the equilibrium constant Kc, and finding equilibrium concentrations.
- Mon Nov 30, 2015 2:49 pm
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: Buffers
- Replies: 2
- Views: 717
Re: Buffers
Buffers generally help stabilize the pH of the solution they are in. So I think that if a buffer was added to strong acid/base solutions, it would still help maintain the pH of those solutions.
- Mon Nov 30, 2015 2:43 pm
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: Buffer mechanics
- Replies: 1
- Views: 664
Re: Buffer mechanics
A buffer system is made up of a weak acid and its conjugate base, or a weak base and its conjugate acid. If a generic weak acid was HA, its conjugate base is A-. - HA reacts with strong bases (such as NaOH) that are added, by donating its proton to form H2O and A- ion. - The A- ion reacts with stron...
- Mon Nov 30, 2015 10:05 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Second Deprotonations of Diprotic Acids
- Replies: 4
- Views: 4935
Re: Second Deprotonations of Diprotic Acids
Generally there is a trend, because as joannali1027 mentioned, after removing one proton (H+) from a polyprotic acid, the molecule has a negative charge (ex. H2PO4- from H3PO4 losing 1 proton). Negative charges attract positive charges, so the negatively charged molecule is more unlikely to let go o...
- Sun Nov 29, 2015 7:17 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Equilibrium Constant
- Replies: 7
- Views: 1272
Re: Equilibrium Constant
Yes, because their product is the constant Kw.
- Sun Nov 29, 2015 7:05 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Conjugate Seesaw
- Replies: 1
- Views: 1359
Re: Conjugate Seesaw
Acids produce conjugate bases when they dissociate in aqueous solution. For example, HCl(aq) + H2O(l) --> Cl-(aq) + H3O+(aq). The conjugate base is Cl- because it is an ion produced from the acid's dissociation, and it can accept protons to produce the acid again (although HCl is so strong that its ...
- Sun Nov 29, 2015 6:41 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Fundementals J15 NaC6H5O is acid/base?
- Replies: 4
- Views: 4006
Re: Fundementals J15 NaC6H5O is acid/base?
C6H5O- is a base because it accepts protons, not donates them. Since phenol is a weak acid, it would not produce C6H5O- as easily, and its dissociation reaction requires an equilibrium symbol: C6H5OH(aq) + H2O(l) <--> C6H5O-(aq) + H3O+(aq) C6H5O- would also be readily converted back to C6H5OH, makin...
- Sun Nov 29, 2015 6:30 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Electron withdrawing effect
- Replies: 1
- Views: 585
Re: Electron withdrawing effect
The electron withdrawing effect is true for oxoacids. For example Cl-O-H is stronger than Br-O-H because Cl, being more electronegative than Br, draws electron density towards itself and stabilizes the negative charge on O. (So electronegativity would cause a greater electron withdrawing effect.) Th...
- Sun Nov 29, 2015 6:11 pm
- Forum: Ionic & Covalent Bonds
- Topic: More than Octect
- Replies: 5
- Views: 1357
Re: More than Octect
Nonmetals starting in group 3, like P, S, and Cl, have empty d orbitals that can be filled with extra electrons, giving them an expanded octet. So S can gain more than 8 electrons because it has spare 3d orbitals to accommodate them.
- Sun Nov 29, 2015 6:08 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configurations for ions
- Replies: 4
- Views: 1002
Re: Electron configurations for ions
4s electrons are lost first only from group 1 and 2 metals like K and Ca, because there are no electrons in 3d orbitals. But with d-block transition metals, as the 3d orbitals gain electrons they become lower energy than 4s. Cr is [Ar]3d 5 4s 1 and Cu is [Ar]3d 10 4s 1 because they are more stable w...
- Sun Nov 29, 2015 5:55 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Fundamental E11
- Replies: 3
- Views: 2496
Re: Fundamental E11
To find the average molar mass of an element from the percentage of its isotopes, you would multiply the percentages (in decimals) by their respective molar masses. In (a), only the masses of 6 Li and 7 Li atoms are given, so you would need to convert them to g/mol. Multiply each mass value by 6.022...
- Sun Nov 29, 2015 5:44 pm
- Forum: Conjugate Acids & Bases
- Topic: Percentage Deprotonated
- Replies: 3
- Views: 987
Re: Percentage Deprotonated
The textbook does this assuming that [H3O+]=[A-], because according to the standard weak acid dissociation equation, HA(aq) + H2O(l) <--> H3O+(aq) + A-(aq), the mole ratio of H3O+ and A- is 1:1. Thus H3O+ and A- would be interchangeable in the deprotonation equation.
- Sun Nov 29, 2015 5:21 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating a pH from a Concentration
- Replies: 2
- Views: 1245
Re: Calculating a pH from a Concentration
For part (b), write the dissociation reaction for HClO4 in water: HClO4(aq) + H2O(l) --> H3O+(aq) + ClO4-(aq).
Because the mole ratio of HClO4 to H3O+ is 1:1, [H3O+]=6.0x10-5 M. Then use pH=-log[H3O+] to get your answer.
Because the mole ratio of HClO4 to H3O+ is 1:1, [H3O+]=6.0x10-5 M. Then use pH=-log[H3O+] to get your answer.
- Sun Nov 29, 2015 2:22 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Problem 12.65
- Replies: 1
- Views: 547
Re: Problem 12.65
The compounds involved are salts, which dissociate in water. For example, NH 4 Br would become NH 4 + and Br - in water. NH 4 + is the only ion that reacts with water, donating a proton to form H 3 O + ions in solution. Br - is too weak (the conjugate base of HBr) to have any reaction, and it appear...
- Sun Nov 29, 2015 1:30 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Phenol vs. 2,4,6-trichlorophenol strength
- Replies: 1
- Views: 3989
Phenol vs. 2,4,6-trichlorophenol strength
How would you account for the fact that 2,4,6-trichlorophenol is a stronger acid than phenol, if only given their Lewis structures and not their Ka values? Phenol: https://upload.wikimedia.org/wikipedia/commons/thumb/9/91/Phenol-2D-skeletal.png/80px-Phenol-2D-skeletal.png 2,4,6-trichlorophenol: http...
- Sun Nov 29, 2015 1:03 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: weak acids and bases
- Replies: 1
- Views: 1153
Re: weak acids and bases
The stronger the acid, the higher the Ka. The reverse is true for pKa: the stronger the acid, the lower the pKa. So according to the given values, HIO3 is the stronger acid because it has the lower pKa. HIO3 is stronger because in an oxoacid, when there are more O atoms bonded to the central atom (t...
- Sun Nov 29, 2015 12:56 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating pH of a very weak acid
- Replies: 1
- Views: 621
Re: Calculating pH of a very weak acid
I think you would generally assume [H3O+]<10 -7 as a guideline to consider the solution neutral. Page 145 of the course reader also says that autoprotolysis of water results in 10 -7 M H3O+. I also found a website that might be of some help: the Dilute Weak Acids and Bases section of http://chemwiki...
- Sun Nov 29, 2015 12:34 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Determining relative strength of acids and bases
- Replies: 4
- Views: 10300
Re: Determining relative strength of acids and bases
CH3NH3Cl is an ionic compound, consisting of CH3NH3+ and Cl- ions. The overall salt does not donate protons, the CH3NH3+ ion does (to form H3O+) when the salt is dissociated in water. Cl- is a very weak conjugate base so its basicity is negligible. Therefore the salt is acidic because of CH3NH3+, a ...
- Sun Nov 29, 2015 12:22 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Homework Question 12.55
- Replies: 1
- Views: 1616
Re: Homework Question 12.55
For (a), you would write the dissociation reaction (with the equilibrium arrows) for CH3COOH in water, then do an ICE box for CH3COOH, CH3COO- and H3O+, with initial [CH3COOH] = 0.20 M. The change would be -x, +x and +x respectively because everything is at a mole ratio of 1:1. Then you would set up...
- Sun Nov 29, 2015 11:58 am
- Forum: Bronsted Acids & Bases
- Topic: Net ionic equations
- Replies: 2
- Views: 671
Re: Net ionic equations
From what I remember, the fundamentals problems with net ionic equations involved salts which dissociate in water. So for those, you would have to recognize that a compound is made of positive and negative ions. For example, NH 4 I contains NH 4 + and I - ions. But there is a guideline sheet for rec...
- Sun Nov 29, 2015 11:56 am
- Forum: Bronsted Acids & Bases
- Topic: Stong Acid/Base
- Replies: 2
- Views: 990
Re: Stong Acid/Base
You can also find a list of common acids and bases (including strong/weak acids and bases) on page 151 of the course reader.
- Sun Nov 29, 2015 11:54 am
- Forum: Bronsted Acids & Bases
- Topic: Identifying Bronsted acids and bases
- Replies: 1
- Views: 710
Re: Identifying Bronsted acids and bases
You would need to separate out the ions by writing a net ionic equation. The (am) subscript means the reaction occurs in ammonia solution, but the ions dissociate the same way. NH 4 I, KNH 2 and KI are all ionic compounds. So you would write NH 4 + (am) + I - (am) + K + (am) + NH 2 - --> K + (am) + ...
- Sat Nov 28, 2015 9:24 pm
- Forum: Amphoteric Compounds
- Topic: determining if a compound is amphoteric
- Replies: 1
- Views: 1670
Re: determining if a compound is amphoteric
Acidity and basicity would vary with the definition you use. According to the Arrhenius definition: - acids produce H+ in aqueous solution - bases produce OH- in aqueous solution. For the Bronsted-Lowry definition, - acids are proton donors - bases are proton acceptors. For the Lewis definition, -ac...
- Sat Nov 28, 2015 8:44 pm
- Forum: Bronsted Acids & Bases
- Topic: Differences between theories
- Replies: 1
- Views: 596
Re: Differences between theories
According to the Arrhenius theory, acids produce H+ ions in solution while bases produce OH- ions in solution. For the Bronsted-Lowry theory, acids donate protons while bases accept protons. But for compounds like NaOH, the Arrhenius theory would be more relevant because it dissociates to form Na+ a...
- Sat Nov 28, 2015 8:38 pm
- Forum: *Titrations & Titration Calculations
- Topic: Volume added at stoichiometric point
- Replies: 2
- Views: 742
Re: Volume added at stoichiometric point
The halfway point of a titration is when the concentrations of the acid and its conjugate base are equal, or the pKa = pH. I assume that by "volume added at the stoichiometric point" you mean the volume added to reach the stoichometric point? Then you could follow the method in page 163 of...
- Sat Nov 28, 2015 8:28 pm
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: Question about Buffers and Use of a Salt
- Replies: 4
- Views: 1052
Re: Question about Buffers and Use of a Salt
Salts with conjugate acids/bases are used because these conjugates are usually in the form of ions and would not be found freely in solution (unlike the weak acid/base which is already a compound). When a salt with the required conjugate acid/base ion is mixed with the buffer solution, it would diss...
- Sat Nov 28, 2015 8:18 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: What is considered a heavier alkaline earth base?
- Replies: 1
- Views: 861
Re: What is considered a heavier alkaline earth base?
Alkaline earth metals past calcium are considered heavier because of their molar mass. The most likely reason for radium not being part of the base-forming metals is its reactivity; its compounds are very short-lived and unstable. Beryllium and magnesium are lighter in comparison, but beryllium form...
- Sat Nov 28, 2015 8:03 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Determining pH
- Replies: 3
- Views: 1253
Re: Determining pH
You would not need to use the ICE box method. Just write the dissociation reactions for each compound in water; then you will be able to find [H 3 O + ] from its mole ratio with the acid, and thus the pH. For instance, in (a), you could write HCl(aq) + H 2 O (l) --> H 3 O + (aq) + Cl - (aq). Because...
- Sat Nov 28, 2015 7:55 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Second Deprotonations of Diprotic Acids
- Replies: 4
- Views: 4935
Re: Second Deprotonations of Diprotic Acids
If Ka2 is significantly less than Ka1, that means the concentration of products is less than that of the reactants for the Ka2 compared to Ka1. In other words, the second deprotonation of the acid is not favored and thus unlikely to occur.
- Fri Nov 27, 2015 9:18 pm
- Forum: Amphoteric Compounds
- Topic: Zinc oxide vs Zinc hydroxide amphoteric character
- Replies: 1
- Views: 2231
Zinc oxide vs Zinc hydroxide amphoteric character
Are zinc oxide and zinc hydroxide both amphoteric compounds or can they act as bases? If so, under what circumstances? Would they be strong or weak bases?
In Fundamentals J.7 part b, zinc hydroxide reacts with nitrous acid to produce zinc nitrite. Could zinc oxide do the same thing as well?
In Fundamentals J.7 part b, zinc hydroxide reacts with nitrous acid to produce zinc nitrite. Could zinc oxide do the same thing as well?
- Fri Nov 27, 2015 8:17 pm
- Forum: *Biological Importance of Buffer Solutions
- Topic: Biologically important buffers
- Replies: 2
- Views: 1998
Biologically important buffers
For the final, will we need to know examples of biologically important buffers and how they work?
- Fri Nov 27, 2015 8:08 pm
- Forum: Bond Lengths & Energies
- Topic: Dissociation Theory
- Replies: 2
- Views: 1274
Re: Dissociation Theory
Dissociation energy is the energy required to break a bond, which is always positive. The shorter the bond, the higher the dissociation energy (because it becomes harder to break shorter/stronger bonds). In the course reader, examples are bonds in F 2 , O 2 and N 2 , which increase progressively in ...
- Fri Nov 27, 2015 7:53 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Order Meaning
- Replies: 3
- Views: 10170
Re: Bond Order Meaning
Because there are only two atoms in a diatomic molecule there cannot be resonance. A fractional bond order such as 1.5 indicates that the molecule in question is less stable than another with a higher bond order like 2. However, it would still be more stable than a molecule with a BO of 1, which has...
- Fri Nov 27, 2015 7:48 pm
- Forum: Bond Lengths & Energies
- Topic: Bonding Orbitals versus Anti-Bonding Orbitals
- Replies: 2
- Views: 837
Re: Bonding Orbitals versus Anti-Bonding Orbitals
In addition, electrons in antibonding MO produce more repulsions than those in bonding MO, making the molecule less stable.
- Fri Nov 27, 2015 7:46 pm
- Forum: Bond Lengths & Energies
- Topic: Stronger Ionic/Covalent Bonds
- Replies: 3
- Views: 1349
Re: Stronger Ionic/Covalent Bonds
Generally the closer you get to F (the most reactive/electronegative atom) the more electronegative a given element would be. The farther you get from F the less electronegative the given atom (except H which is much more electronegative than the group 1 metals). So PF5 would have a stronger covalen...
- Fri Nov 27, 2015 7:35 pm
- Forum: Bronsted Acids & Bases
- Topic: KOH as a Bronsted base
- Replies: 1
- Views: 4800
KOH as a Bronsted base
It's fairly clear that KOH is a base because it is an alkaline metal (first group) hydroxide and it dissociates completely in water. But when writing its reaction in water (KOH + H2O --> K+ + OH- + H2O), how would that show whether KOH accepts a proton as a Bronsted base?
- Fri Nov 27, 2015 7:29 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted and Lewis Acids and Bases
- Replies: 3
- Views: 911
Re: Bronsted and Lewis Acids and Bases
If you look at the compound's reaction in water you should be able to see whether it accepts or donates a proton. For example, NH3 in water produces NH4+ and OH- ions; because NH3 accepted a proton from water, it is a Bronsted base. It would also be a Lewis base because it technically "donated&...
- Fri Nov 27, 2015 3:08 pm
- Forum: Naming
- Topic: using bis, tris, tetrakis
- Replies: 4
- Views: 13270
Re: using bis, tris, tetrakis
You would use bis-, tris-, and tetrakis- when the ligand already has prefixes like di- and tri- in its name, or if you know for sure it is polydentate (includes bidentate, tridentate, hexadentate or generally anything over monodentate). If you were naming 2 dien ligands, you would write bis(dien) or...
- Fri Nov 27, 2015 2:51 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Solids and Water Vapor
- Replies: 2
- Views: 1556
Re: Solids and Water Vapor
Adding solids does not affect the equilibrium, because Kc only includes aqueous and/or gas components, and Kp only includes gases. Also Le Chatelier's principle applies to concentration, pressure and temperature changes. When the concentrations of products/reactants changes, the reaction shifts in o...
- Sun Nov 22, 2015 6:42 pm
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 157074
Re: Reading the textbook
The text uses some different symbols from the ones we use and the equations are more advanced which can be intimidating, but it has a lot of useful background information in the actual text portion. The bolded words are also relevant most of the time.
- Sun Nov 22, 2015 6:40 pm
- Forum: Ideal Gases
- Topic: gas constant R
- Replies: 18
- Views: 1773
Re: gas constant R
The units of pressure would need to be given. If pressure is in atm, then use R = 0.08206 L*atm/K*mol to cancel out. If pressure is in bar, the unit for R would be L*bar/K*mol, and for torr it is L*torr/K*mol.
- Mon Nov 16, 2015 9:31 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sulfato denticity
- Replies: 1
- Views: 673
Sulfato denticity
Sulfato is listed as generally monodentate but can also be bidentate. Under what circumstances would it be the latter?
- Mon Nov 16, 2015 8:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Determining chelating ligands from structure
- Replies: 1
- Views: 346
Determining chelating ligands from structure
How can you tell if a "ring" is formed around the central metal atom as is characteristic of chelating ligands? Can this be determined from the Lewis structure or is the molecular shape necessary? In other words is the ring usually three dimensional? Porphyrins don't seem to do this accord...
- Mon Nov 16, 2015 9:36 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Number of bonds TM can make
- Replies: 1
- Views: 379
Number of bonds TM can make
How can you tell the number of bonds a transition metal can make, based on its oxidation number? Or will it only be necessary to determine the coordination number from the formula of the coordination complex?
- Sun Nov 15, 2015 2:06 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: EDTA number of bonds
- Replies: 1
- Views: 602
EDTA number of bonds
In the course reader EDTA is listed as a hexadentate molecule, but could it also be tetradentate? If so, when would each of these forms occur? Do they have anything to do with the charge on the EDTA molecule?
- Sun Nov 15, 2015 8:03 am
- Forum: Naming
- Topic: Determining cis or trans forms from names
- Replies: 1
- Views: 258
Determining cis or trans forms from names
Is it possible to determine cis or trans forms of coordination compounds from their names (or if we write the formula from the name)? Would this be necessary on quiz 3?
- Fri Nov 13, 2015 9:19 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Conditions favoring production - pressure
- Replies: 6
- Views: 1167
Re: Conditions favoring production - pressure
So what would happen if inert gases were added to the reaction? Would that have no effect on the equilibrium whatsoever?
- Tue Nov 10, 2015 10:34 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Conditions favoring production - pressure
- Replies: 6
- Views: 1167
Conditions favoring production - pressure
For the equation 2SO 3 (g) <---> 2SO 2 (g) + O 2 (g) which requires heat, it makes sense that increasing the temperature would favor SO 2 production. But why is it that low pressure (instead of high) also favors production of SO 2 ? Is it related to how many types of gases are present on each side o...
- Tue Nov 10, 2015 10:11 am
- Forum: Naming
- Topic: Adding -ate at the end of names
- Replies: 2
- Views: 604
Adding -ate at the end of names
The course reader states that -ate should be added at the end of the metal name if the complex has a negative charge, and shows an example with a charge at the end. Does this rule also apply when there are cations attached to the complex to cancel out the charge? Also do we just write the metal name...
- Tue Nov 10, 2015 10:01 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Bar unit of pressure
- Replies: 1
- Views: 569
Bar unit of pressure
In the workbook an equilibrium problem listed the pressures of three gases as 1.08 bar, 4.9 bar and 7.6 bar, but this unit isn't mentioned in the course reader. Do need to know it for the quiz, and how do we convert it to other units like torr and atm?
- Tue Nov 10, 2015 9:31 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When to use Kp or Kc for gases
- Replies: 1
- Views: 3083
When to use Kp or Kc for gases
In the course reader Kc is used for most of the icebox examples involving gases. Would we ever need to use Kp in an equilibrium problem? When is Kp generally used in problems with gases? Also, when asked to write expressions for equilibrium constants, would writing either Kp or Kc be acceptable for ...
- Tue Nov 10, 2015 9:22 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Cis and trans forms of coordination compounds
- Replies: 1
- Views: 807
Cis and trans forms of coordination compounds
For coordination compounds, do we need to know how to differentiate between cis and trans forms of the same compound? If so, how would we do this?
- Tue Nov 10, 2015 9:19 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Writing oxidation numbers for transition metals
- Replies: 2
- Views: 682
Writing oxidation numbers for transition metals
When writing oxidation numbers for transition metals, do we write them with the sign first? For instance, if cobalt has an oxidation number of 3, would we write it as 3+ or +3? Is there any difference between the two?
- Tue Nov 03, 2015 7:57 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Orbital arrangement for diatomic molecules involving F
- Replies: 1
- Views: 319
Orbital arrangement for diatomic molecules involving F
In the diatomic molecule HF, the fluorine atom F holds its atomic orbitals closer than most atoms, so its 2s and 2p orbitals are lower energy than the hydrogen's 1s orbital. For other diatomic molecules involving F, is this the same case? On another note, would we need to draw MO diagrams for ionic ...
- Mon Oct 26, 2015 10:29 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Pi orbitals
- Replies: 1
- Views: 263
Pi orbitals
When determining the MO diagram, why are the \pi orbitals divided into \pi _{x} and \pi _{_{y}} and their corresponding antibonding components? In lecture Professor Lavelle said that the \sigma _{z} was for sigma bonding on the z axis. So are the pi bonding orbitals also divided according to bonds f...
- Fri Oct 23, 2015 9:25 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: How to distinguish between bonding/antibonding orbitals
- Replies: 1
- Views: 610
How to distinguish between bonding/antibonding orbitals
How do you determine bonding and antibonding orbitals and which electrons go into the sigma and pi orbitals?
- Mon Oct 19, 2015 8:45 am
- Forum: Bond Lengths & Energies
- Topic: Number of bonds and bond length
- Replies: 1
- Views: 869
Number of bonds and bond length
Does the number of a particular bond type formed by a central atom affect the average bond length?
For example in the textbook question 85b, both SO3 and SO2 have all double bonds. Would the fact that S formed more double bonds in SO3 make the average bond length shorter in SO3?
For example in the textbook question 85b, both SO3 and SO2 have all double bonds. Would the fact that S formed more double bonds in SO3 make the average bond length shorter in SO3?
- Mon Oct 19, 2015 8:42 am
- Forum: Lewis Structures
- Topic: Lewis Structure of P4
- Replies: 1
- Views: 13818
Lewis Structure of P4
What is the correct Lewis structure for P4 (white phosphorus)?
- Sun Oct 18, 2015 3:39 pm
- Forum: Lewis Structures
- Topic: What is the correct Lewis structure for BrO+?
- Replies: 3
- Views: 2390
Re: What is the correct Lewis structure for BrO+?
Why is the Lewis structure double bonded instead of triple bonded? Does it matter which atom has the formal charge?
- Sun Oct 18, 2015 3:35 pm
- Forum: Lewis Structures
- Topic: K3P Lewis structure
- Replies: 2
- Views: 4268
Re: K3P Lewis structure
Do you know which way of drawing it would be most correct? I thought the first one might have implied covalent bonding since it used lines.
- Fri Oct 16, 2015 8:28 pm
- Forum: Lewis Structures
- Topic: K3P Lewis structure
- Replies: 2
- Views: 4268
K3P Lewis structure
When writing the Lewis structure of the ionic compound 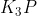 , or any similar ionic compound, how should the cations be placed around the anion?
, or any similar ionic compound, how should the cations be placed around the anion?
- Fri Oct 16, 2015 8:23 pm
- Forum: Lewis Structures
- Topic: Lewis Structures - Lone Pairs
- Replies: 1
- Views: 412
Lewis Structures - Lone Pairs
If an atom in a Lewis structure has at least two lone pairs, as in NH2-, where should the lone pairs be located? Should we take into account molecular shape when locating the lone pairs?
- Fri Oct 16, 2015 8:20 pm
- Forum: Lewis Structures
- Topic: Orientation of Atoms in Lewis Structures
- Replies: 2
- Views: 1133
Orientation of Atoms in Lewis Structures
When drawing a Lewis structure where a central atom is surrounded by at least two different types of atoms, does it matter how the structure is oriented? For example, with COCl2, can the positions of Cl and O vary as long as the bonds and lone pairs are correct?
- Mon Oct 12, 2015 4:16 pm
- Forum: Octet Exceptions
- Topic: Expanded octets - all atoms with 3d orbitals?
- Replies: 1
- Views: 856
Expanded octets - all atoms with 3d orbitals?
P, S, and Cl can have expanded octets because they have extra 3d orbitals. Can this also happen with any atom in higher periods, since they will have 3d orbitals too? Do metals like Al, K, Ca and the transition metals follow the octet rule exactly?
- Fri Oct 09, 2015 8:56 pm
- Forum: Empirical & Molecular Formulas
- Topic: Determination of oxoanions - what makes an ion an oxoanion?
- Replies: 1
- Views: 451
Determination of oxoanions - what makes an ion an oxoanion?
In the course reader oxoanions "are polyatomic anions that contain oxygen" and includes ions like carbonate. Does this mean carbonic acid would then be an oxoacid? Also, would acetic acid be considered an oxoacid, since acetate also contains oxygen? If not, what is the criteria for determi...

