Search found 12 matches
- Sat Mar 12, 2016 8:12 pm
- Forum: *Cycloalkanes
- Topic: Phenol groups
- Replies: 1
- Views: 457
Phenol groups
Are we going to required to know phenol groups? I know they're important in organic chemistry but we never went over them in lecture.
- Sat Mar 12, 2016 8:09 pm
- Forum: *Electrophiles
- Topic: General Question about Nucleophiles vs. Electrophiles
- Replies: 2
- Views: 1606
General Question about Nucleophiles vs. Electrophiles
In quiz 3, they gave us several molecules (e.g. CH3+, OH-, CH3,Cl, CO2) and asked us to categorize them either as an electrophile or nucleophile. Other than the overall charge of the molecule, is there another way to tell whether a molecule is more electrophillic/nucleophillic?
- Sat Mar 12, 2016 8:00 pm
- Forum: General Rate Laws
- Topic: Question from Quiz 2
- Replies: 1
- Views: 421
Question from Quiz 2
The question gives that A + B + C --> X and the rate law would be R=k[A]^2[B][C] and it asks you to find the rate constant k for the reaction given that the excess reactants concentration of A and B is [A]_o_ [B]_o_ How do you solve for k with this given. I had to leave this one blank on the quiz be...
- Sat Mar 12, 2016 7:52 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Midterm Question
- Replies: 1
- Views: 404
Midterm Question
IN the midterm, question 8, the test asks what would the effect on the potential of the cell if the size of the silver electrode were doubled?
I understand that nothing would happen, but why would there be no effect on E?
I understand that nothing would happen, but why would there be no effect on E?
- Sun Feb 21, 2016 11:00 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3674270
Re: Chemistry Jokes
In a sports relay race, a chemical kinetics specialist runs slowly, and his group loses the race.
When the chemical kinetics specialist is asked why he ran slowly, his reply was “I always wanted to be the significant rate determining step”.
When the chemical kinetics specialist is asked why he ran slowly, his reply was “I always wanted to be the significant rate determining step”.
- Sun Feb 07, 2016 7:49 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: HW 14.11 Part D
- Replies: 1
- Views: 332
HW 14.11 Part D
In the problem, O2 and H+ are on the anode but they are being combined to form water, why is this? Typically the two molecules would exchange electrons on separate sides of the chemical reaction.
- Fri Feb 05, 2016 12:52 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: HW 11.11
- Replies: 1
- Views: 362
HW 11.11
When solving the question, you should use 
If represents the Gibbs Free Energy at STP, why does the solutions manual solve for
represents the Gibbs Free Energy at STP, why does the solutions manual solve for  at 1200K rather than 298K?
at 1200K rather than 298K?
If
- Sun Jan 31, 2016 9:11 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 3
- Views: 596
Bond Enthalpies
What's the difference between bond enthalpies and enthalpies of formation and can you use either to solve 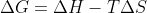
- Mon Jan 18, 2016 3:54 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Enthalpy of Vaporization
- Replies: 1
- Views: 403
Enthalpy of Vaporization
When doing the homework problems for chapter 8 (I believe the question in the book is either 35 or 37) it asks for the Enthalpy of Vaporization. What is the formula for this. Also I noticed when doing the bomb calorimeter problems, the solutions manual uses the formula q = C sp *delta*T... why is th...
- Mon Jan 18, 2016 12:01 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Question 8.1
- Replies: 1
- Views: 480
Question 8.1
Why would coffee in a thermos be considered an isolated system? In my everyday experience with thermoses, the coffee would cool if put in the fridge, and thus have a change in energy.
- Sun Nov 29, 2015 2:17 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lewis Structures and VSEPR
- Replies: 2
- Views: 581
- Sat Nov 28, 2015 11:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lewis Structures and VSEPR
- Replies: 2
- Views: 581

