Search found 9 matches
- Fri Mar 11, 2016 5:19 pm
- Forum: *Cyclopropanes and Cyclobutanes
- Topic: Eclipse
- Replies: 1
- Views: 1447
Eclipse
When we say that a cyclohexane is eclipsed, should we say all the carbon-carbon bonds are eclipsed, or Carbon-hydrogen bonds are eclipsed?
- Sun Feb 28, 2016 7:05 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Useful Summary of Thermodynamic Definitions
- Replies: 55
- Views: 18626
Re: Useful Summary of Thermodynamic Definitions
Thank you!!
- Tue Feb 09, 2016 11:02 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Perfect Crystal
- Replies: 1
- Views: 4473
Perfect Crystal
What is the definition for perfect crystal? and what does professor mean by that there is no impurity and defect?
Also, According to the formula,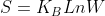 , should the entropy be 0 if W=1, even when the temperature is not 0 degree?
, should the entropy be 0 if W=1, even when the temperature is not 0 degree?
Also, According to the formula,
- Sun Feb 07, 2016 11:49 pm
- Forum: Balancing Redox Reactions
- Topic: Anode and Cathode
- Replies: 3
- Views: 753
Re: Anode and Cathode
The reactant in the reaction that has the lower cell potential will give out electron in the whole electrochemical reaction (aka being oxidized), and become the anode.
- Wed Jan 27, 2016 8:31 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Residual Entropy
- Replies: 5
- Views: 1920
Re: Residual Entropy
Then how do we know the number of possible orientation?
- Sun Jan 17, 2016 11:24 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam Burns vs. Water Burns
- Replies: 6
- Views: 4409
Re: Steam Burns vs. Water Burns
It requires energy to turn water from a room temperature to 100 degrees (the boiling point), and it also requires energy to turn boiling water to steam. To convert one gram of water to steam requires 540 calories. Thus steam stores more energy than water, thus causes more damage.
- Fri Dec 04, 2015 7:22 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Why is H-H Equation an approximation
- Replies: 2
- Views: 1400
Why is H-H Equation an approximation
I think I heard prof say Henderson-Hasselbalch Equation is a way to get the approximate pH. I didn't get why. Would anybody explain?
Also why the beginning of the pH curve of a strong acid-strong base titration flatter than a strong acid-weak base titration?
Thank you.
Also why the beginning of the pH curve of a strong acid-strong base titration flatter than a strong acid-weak base titration?
Thank you.
- Fri Nov 20, 2015 11:49 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Methane classification
- Replies: 3
- Views: 870
Re: Methane classification
I'd say methane is a very weak acid because it is a proton donor but can be ionized at a low level.
- Sun Oct 25, 2015 5:58 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Energy Change When Losing a Electron
- Replies: 2
- Views: 734
Energy Change When Losing a Electron
From the book we know that removing a e- requires energy, so does it mean after the e- is moved, the energy of the atom(ion) will increase?

