Search found 42 matches
- Thu Mar 10, 2016 10:37 pm
- Forum: *Electrophilic Addition
- Topic: The Number of Intermediates for Electrophilic Addition
- Replies: 3
- Views: 751
Re: The Number of Intermediates for Electrophilic Addition
Dr. Lavelle said that anything between the reactant and product is considered an intermediate. That is how I approach it.
- Sun Mar 06, 2016 6:36 pm
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Cis and trans in line structures
- Replies: 1
- Views: 530
Re: Cis and trans in line structures
Take the page and turn it slightly to the right. From the carbon-carbon double bond, there a C and H on the same side. The C has higher priority than H, making is trans- or E-.
- Sun Mar 06, 2016 6:31 pm
- Forum: General Science Questions
- Topic: Ketones
- Replies: 1
- Views: 822
Re: Ketones
I believe so. The different endings are only because of the functional groups for each molecule, and the numbers in the middle of the parent chain represent the location of single/double bonds.
Re: Naming
Start with the parent chain and then focus on the number of bonds, functional groups, and substituents.
Remember to keep the numbers as low as possible!
Remember to keep the numbers as low as possible!
- Sun Mar 06, 2016 6:27 pm
- Forum: *Cyclohexanes (Chair, Boat, Geometric Isomers)
- Topic: Torsional & Steric Strain
- Replies: 2
- Views: 720
Re: Torsional & Steric Strain
Torsional strain is developed from the electron activity surrounding each atom. When the molecule is eclipsed, the electrons are being forced together, and they naturally repel. Steric strain is developed from physical interactions. This is when the actual atoms are hitting each other due to the clo...
- Thu Mar 03, 2016 11:05 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: The A in the Arrhenius Equation
- Replies: 1
- Views: 504
Re: The A in the Arrhenius Equation
Once the equation is written out, they should cancel out. I believe there is an example in the course reader.
- Thu Mar 03, 2016 11:04 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts in relation to Graph Peaks
- Replies: 2
- Views: 593
Re: Catalysts in relation to Graph Peaks
When a catalyst is present, there is a lower activation energy because the reaction is sped up.
- Thu Mar 03, 2016 9:32 am
- Forum: *Identifying Primary, Secondary, Tertiary, Quaternary Carbons, Hydrogens, Nitrogens
- Topic: Iso and neo vs. sec and tert
- Replies: 2
- Views: 924
Re: Iso and neo vs. sec and tert
For sec- the center carbon should be attached to two other carbons as well.
- Thu Mar 03, 2016 9:29 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: How to solve with K without an A value?
- Replies: 3
- Views: 801
Re: How to solve with K without an A value?
When you write out the division problem, the A's should cancel out.
- Thu Mar 03, 2016 9:28 am
- Forum: *Organic Reaction Mechanisms in General
- Topic: Which carbon in a double bond does the electrophile bond to
- Replies: 3
- Views: 675
Re: Which carbon in a double bond does the electrophile bond
As long the molecule is symmetrical, you can attach it to the either carbon. The halogen then attaches to the carbocation.
- Sun Feb 21, 2016 7:06 pm
- Forum: *Nucleophiles
- Topic: Nucleophile Example in the OTextbook
- Replies: 1
- Views: 552
Re: Nucleophile Example in the OTextbook
Nucleophiles meet these standards:
1) Negative Charge
2) Double Bonds (such as the hydrocarbon mentioned)
3) Extra lone pairs
1) Negative Charge
2) Double Bonds (such as the hydrocarbon mentioned)
3) Extra lone pairs
- Sun Feb 21, 2016 6:57 pm
- Forum: *Nucleophiles
- Topic: Intermediate Line
- Replies: 2
- Views: 638
Re: Intermediate Line
The line has to be even with the reactants because is the reference point. Think of it as sea level. Anything higher has a positive change, and anything below has a negative change.
- Mon Feb 15, 2016 9:59 pm
- Forum: General Rate Laws
- Topic: graphing reactions
- Replies: 2
- Views: 636
Re: graphing reactions
Correction:
2nd Order is 1/[conc]
2nd Order is 1/[conc]
- Mon Feb 15, 2016 2:53 pm
- Forum: General Rate Laws
- Topic: graphing reactions
- Replies: 2
- Views: 636
Re: graphing reactions
That depends on the order.
You use ln[conc] for first order.
You use 1/ln[conc] for second order
You use [conc] for zero order.
You use ln[conc] for first order.
You use 1/ln[conc] for second order
You use [conc] for zero order.
- Thu Feb 11, 2016 10:43 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Salt Bridge
- Replies: 5
- Views: 1187
Re: Salt Bridge
I was under the impression that they were necessary. I have not seen an example that does not include one. The salt does not affect the reaction, and it prevents electron build up (as mentioned above).
- Sun Feb 07, 2016 10:33 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Ideal Gases
- Replies: 1
- Views: 531
Re: Ideal Gases
These are how you calculate the heat capacity of ideal gases.
You use the 3/2R equation for monatomic gases.
You use the 5/2R equation for diatomic gases.
You use the 3/2R equation for monatomic gases.
You use the 5/2R equation for diatomic gases.
- Sun Feb 07, 2016 10:31 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Difference in values of Gibbs Free Energy
- Replies: 2
- Views: 2527
Re: Difference in values of Gibbs Free Energy
I believe it is because H2 is the natural state of Hydrogen, while Br2 is not the natural state of Bromine.
- Thu Feb 04, 2016 8:28 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Reactions with e-
- Replies: 1
- Views: 418
Balancing Redox Reactions with e-
How do you know how many electrons to add?
- Fri Jan 29, 2016 3:04 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Max Potential
- Replies: 1
- Views: 412
Max Potential
How do you calculate the max potential of cells? Why is the concept important?
- Thu Jan 21, 2016 9:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: value of n in w= -nRT*ln(V2/V1) vs. q=nCΔT
- Replies: 2
- Views: 1594
Re: value of n in w= -nRT*ln(V2/V1) vs. q=nCΔT
I believe this is the case simply because you are given the values to solve for n.
- Thu Jan 21, 2016 9:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy
- Replies: 2
- Views: 647
Re: Internal Energy
The change of internal energy does not depend on how the system got to the initial or final state.
- Thu Jan 21, 2016 9:09 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Molar Heat Capacity Formula of Cp and Cv
- Replies: 2
- Views: 1151
Molar Heat Capacity Formula of Cp and Cv
I was just wondering if there were different equations for when the pressure is constant and volume is constant. Or do you use the same equation?
Thank You.
Thank You.
- Wed Jan 13, 2016 9:34 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Rationale for higher heat capacity
- Replies: 1
- Views: 449
Re: Rationale for higher heat capacity
The molar heat capacity higher when the molecule is more complicated. The more bonds a molecule has the more vibrational movements and rotation movements the molecule has. This means that it requires more energy to be raised one degree celsius since the energy is being used in multiple bonds.
- Fri Jan 08, 2016 5:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law, Chapter 8 HW
- Replies: 1
- Views: 404
Hess's Law, Chapter 8 HW
My question is in regards to the chapter 8 homework question 61.
When cancelling the out various components to the reactions, can you cancel any products from any reactants or does it have to be the continuing reaction.
For 61, can I cancel the 2NH4Br in the first and last equation?
Thank you.
When cancelling the out various components to the reactions, can you cancel any products from any reactants or does it have to be the continuing reaction.
For 61, can I cancel the 2NH4Br in the first and last equation?
Thank you.
- Mon Nov 23, 2015 9:29 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis vs Bronsted Acids
- Replies: 3
- Views: 941
Lewis vs Bronsted Acids
How can you tell whether an acid is a Lewis or Bronsted acid? Does it have anything to do with whether the acid is a product or reactant?
I'm really confused on determining this.
Thank you,
Victoria
I'm really confused on determining this.
Thank you,
Victoria
- Thu Nov 19, 2015 12:58 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Chemical Equilibrium #11.63 and Quiz #10 Video
- Replies: 2
- Views: 817
Re: Chemical Equilibrium #11.63 and Quiz #10 Video
I am pretty sure this is for the second quiz in our green book.
- Tue Nov 17, 2015 10:19 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Chemical Equilibrium #11.63 and Quiz #10 Video
- Replies: 2
- Views: 817
Chemical Equilibrium #11.63 and Quiz #10 Video
Hope this helps.
- Fri Nov 13, 2015 11:28 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Determining the Coordination Number
- Replies: 2
- Views: 530
Determining the Coordination Number
How do you determine the coordination number of a central metal ion?
- Wed Nov 11, 2015 3:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Fall 2014 Quiz 3 #8
- Replies: 1
- Views: 377
Fall 2014 Quiz 3 #8
This is the question... Consider the following reaction: NH_{4}HS (s)\rightarrow NH_{3} (g) + H_{2}S (g) If the value of Kp os 0.11 atm^{2} at 300 K, calculate the equilibrium partial pressure of NH_{3} (g) starting from pure NH_{4}HS (s) . I don't know how to approac...
- Mon Nov 09, 2015 9:55 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate
- Replies: 8
- Views: 2181
Re: Polydentate
HN(CH_{2}CH_{2}NH{2})_{2} would be polydentate, more specifically tridentate. Each of the three nitrogens has a lone pair, so those can be replaced by a bond. CO_{3} 2- can be either mono- or polydentate; however, it is more often monodentate. Remember that the molecular shape is trigonal p...
- Mon Nov 09, 2015 9:41 am
- Forum: Naming
- Topic: Chelating Ligands
- Replies: 1
- Views: 348
Re: Chelating Ligands
A chelate is a complex containing a ligand that forms a ring of atoms that includes the central metal atom. This is directly from the course reader on page 104.
A chelate is a closed loop, so it must have at least two ligating atoms.
A chelate is a closed loop, so it must have at least two ligating atoms.
- Sun Nov 01, 2015 10:53 am
- Forum: General Science Questions
- Topic: General Question about MO Diagrams
- Replies: 2
- Views: 1289
Re: General Question about MO Diagrams
I would draw them all just to avoid any confusion. Also, it would be easier for the graders to find partial points to give you.
I did hear that if it ask for all four, you are REQUIRED to do all four.
I did hear that if it ask for all four, you are REQUIRED to do all four.
- Sun Nov 01, 2015 10:52 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Molecular Orbital Theory when nuclear charge is less than 8
- Replies: 1
- Views: 324
Re: Molecular Orbital Theory when nuclear charge is less tha
It takes more energy for them pair in a sigma bond than a pi bond, in this case.
- Sat Oct 24, 2015 3:03 pm
- Forum: Hybridization
- Topic: Quiz 2 Preperation #1 (Fall 2013)
- Replies: 2
- Views: 526
Quiz 2 Preperation #1 (Fall 2013)
After I did the Lewis Structure, I got  , but the answer was just
, but the answer was just 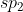 ? Why is this? Should their be a 2 because it is in period 2?
? Why is this? Should their be a 2 because it is in period 2?
- Sat Oct 24, 2015 2:47 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework Question 4.1
- Replies: 1
- Views: 455
Homework Question 4.1
For question 4.1 there is a linear molecule with an angle of 180 degrees. There question asks if there must be, may be, or cannot be one or more lone pairs of electrons on the central atom. I thought the answer would be there cannot be because it is linear, but the solutions manual said their may be...
- Fri Oct 16, 2015 3:40 pm
- Forum: Lewis Structures
- Topic: Dots for Lewis Structures
- Replies: 2
- Views: 1438
Dots for Lewis Structures
Does it matter where you put the dots for the elements while doing a Lewis Structure? Between the two Lewis Structures attached, which one would be right?
- Sun Oct 11, 2015 8:36 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Configurations
- Replies: 1
- Views: 434
Configurations
I am confused on what number means what during an electronic configuration. So, the first number is the energy level and the row number ( and d is one behind)? The letter tells what orbital the electron is in? The number tells how many electrons are in the orbitals and whether they are paired or par...
- Sun Oct 11, 2015 7:36 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Configurations
- Replies: 1
- Views: 502
Configurations
I am confused on what number means what during an electronic configuration. So, the first number is the energy level and the row number ( and d is one behind)? The letter tells what orbital the electron is in? The number tells how many electrons are in the orbitals and whether they are paired or par...
- Mon Oct 05, 2015 6:56 pm
- Forum: Trends in The Periodic Table
- Topic: Orbitals and the Periodic Table
- Replies: 1
- Views: 484
Orbitals and the Periodic Table
During the lecture on 10/5 Professor Lavelle talked about how to you can tell what shape the orbital of an element is based on the periodic table, and I was confused about how this was done exactly. When he described them as blocks, where do they begin and end?
- Fri Oct 02, 2015 2:33 pm
- Forum: Significant Figures
- Topic: All students read this sig fig post [ENDORSED]
- Replies: 170
- Views: 34528
Re: All students read this sig fig post [ENDORSED]
SigFigs are so simple they get confusing, I am just going to stick with the right amount, and if I am skeptical just go with less. My TA said that you can't add precision, but you can make it less precise.
- Fri Oct 02, 2015 2:29 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic spectra
- Replies: 2
- Views: 578
Re: Atomic spectra
I am pretty sure is saying that although a new electron can be emitted, the same one can continue to be emitted, and each time it is emitted it loses more energy.
- Fri Oct 02, 2015 2:28 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation
- Replies: 1
- Views: 665
Rydberg Equation
For chapter 1 question 57, why is it (1/2^2) - 1(7^2) in the Rydberg equation? Since the two is the first number in the Balmer Series, does the sequence start at 2? The question is trying to solve for the fifth line in the sequence, so would it go N1=2 and then N2=3,4,5,6,7 with 7 being the fifth nu...

