Search found 16 matches
- Mon Mar 07, 2016 10:14 am
- Forum: *Alkanes
- Topic: Naming in General
- Replies: 2
- Views: 642
Re: Naming in General
To add to this, "iso" and "neo" should be included when alphabetizing, while "sec" and "tert" are not.
Naming
In naming compounds, which takes priority for the lowest number: a double bond or a functional group?
- Mon Feb 22, 2016 12:02 pm
- Forum: *Alkanes
- Topic: Drawing line structures
- Replies: 1
- Views: 485
Drawing line structures
On a quiz or the final, is it acceptable to leave out the hydrogens when drawing line structures?
- Mon Feb 15, 2016 5:31 pm
- Forum: Zero Order Reactions
- Topic: Rate constant
- Replies: 3
- Views: 778
Re: Rate constant
If given the initial concentration at t=0, then you can calculate k by using the equation 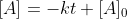
Otherwise, you can calculate k by plotting the concentrations you have vs time and determining the negative of the slope as the equation shows.
Otherwise, you can calculate k by plotting the concentrations you have vs time and determining the negative of the slope as the equation shows.
- Mon Feb 08, 2016 11:59 am
- Forum: General Rate Laws
- Topic: Rate Laws
- Replies: 1
- Views: 398
Rate Laws
Are liquid and solid reactants excluded in the rate law?
- Mon Feb 01, 2016 11:24 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Positive/Negative Voltage
- Replies: 1
- Views: 501
Positive/Negative Voltage
On a quiz or exam, would a negative voltage be acceptable? Or should it always be positive since that would be the favorable reaction?
- Mon Jan 25, 2016 12:11 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive V. Intensive
- Replies: 2
- Views: 730
Re: Extensive V. Intensive
An extensive property is a property that depends on the size of the sample. Heat capacity is an example of an extensive property since heat required depends on the amount of substance you have. On the other hand, an intensive property doesn't change regardless of the sample size. For example, if you...
- Thu Jan 21, 2016 4:00 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3613595
- Wed Jan 13, 2016 4:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Comparing Molar Heat Capacity
- Replies: 1
- Views: 423
Re: Comparing Molar Heat Capacity
NO2 has more atoms in the molecule compared to NO, and thus a higher molecular complexity. Heat capacity increases with molecular complexity. This is because there are additional vibrational modes within more complex molecules, allowing more heat to be absorbed.
- Wed Jan 06, 2016 5:26 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes
- Replies: 2
- Views: 657
Phase Changes
From my understanding, the phase change from water to steam requires more heat than the phase change from ice to water. How would this difference be represented in a heating curve?
- Tue Nov 24, 2015 9:55 pm
- Forum: Naming
- Topic: VIDEO: Naming Coordination Compounds
- Replies: 1
- Views: 482
VIDEO: Naming Coordination Compounds
Submission by: Lanzel Patawaran 304630292, Discussion 3B
- Sun Oct 18, 2015 4:08 pm
- Forum: Lewis Structures
- Topic: Homework 3.97
- Replies: 2
- Views: 586
Re: Homework 3.97
The atom with the lowest ionization energy/electronegativity goes in the middle of the structure. In P4, all the atoms have the same ionization energy/electronegativity, so there is no tendency for one atom to be the central atom.
- Sun Oct 18, 2015 3:55 pm
- Forum: Resonance Structures
- Topic: Electron Heteronuclear Covalent Bond
- Replies: 1
- Views: 469
Re: Electron Heteronuclear Covalent Bond
Electron heteronuclear covalent bonds are covalent bonds between two different types of atoms. For example, O2 is not heteronuclear because it contains two of the same atoms, whereas CO is heteronuclear because it contains two different atoms.
- Sun Oct 11, 2015 10:59 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Chapter 2, Problem #29
- Replies: 1
- Views: 511
Re: Chapter 2, Problem #29
First of all, these are the values that the quantum numbers can take: n: any positive integer l: n-1 m(l): -l < m(l) < 1 -l \leq m_{l} \leq l m(s): -1/2 or 1/2 For part b, you are already given n, l, and m(l). This means that m(s) can either be -1/2 or 1/2. So, only 2 electrons can have the quantum ...
- Sun Oct 11, 2015 10:50 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Shielding of Electrons?
- Replies: 1
- Views: 502
Re: Shielding of Electrons?
Orbitals with higher l values are NOT better at shielding than orbitals with lower l values. Since the electrons in atoms with lower l values are on average closer to the nucleus (like the s orbital compared to the p orbital), they cancel out the positive charge of the nucleus, making the effective ...
- Sun Oct 04, 2015 3:11 pm
- Forum: Einstein Equation
- Topic: HW 1.27
- Replies: 1
- Views: 599
Re: HW 1.27
The energy of light for a single photon is E=hv, where h is planck's constant and v is the frequency of the light/photon. Since power is how much energy is transferred per second, the power must be proportional to the number of photons emitted per second, n. Since each of these photons has an energy...

