Search found 21 matches
- Fri Mar 11, 2016 8:51 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 2013 winter Q3
- Replies: 1
- Views: 489
Re: 2013 winter Q3
That's because n is equal to the number of electrons transferred which when we look at the balanced half reactions, was 6.
- Fri Mar 11, 2016 8:44 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 2009 final 4E
- Replies: 1
- Views: 435
Re: 2009 final 4E
I think it's because we're looking for pH and the concentrations of Ce3+, Ce4+,MnO4-, and Mn2+ would not contribute to pH because pH is the measure in concentration of H+ so that is what we could focus on instead. So by taking the E (which would be .157) you can divide that by (.05916/5) and then di...
- Fri Mar 04, 2016 4:26 pm
- Forum: *Alkanes and Substituted Alkanes (Staggered, Eclipsed, Gauche, Anti, Newman Projections)
- Topic: Staggered Conformations vs Eclipsed
- Replies: 1
- Views: 467
Staggered Conformations vs Eclipsed
Is there any other reason besides collision that would explain why the molecules would not just stay in the staggered conformation?
- Tue Feb 23, 2016 5:27 pm
- Forum: *Alkanes
- Topic: Naming Compounds Clarification
- Replies: 1
- Views: 485
Naming Compounds Clarification
For the first quiz 3 prep, the answer for question 2 listed the name for CH3CH(CH3)CH(CH2CH3)C(CH3)3 as 2,2,4-trimethyl-3-ethylpentane. Why isn't it 3-ethyl-2,2,4-trimethyl-pentane, having ethyl come before methyl? Also if there are multiple substituents, would the numbers change so the first substi...
- Fri Feb 12, 2016 8:25 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Practice Quiz winter 2015
- Replies: 1
- Views: 494
Practice Quiz winter 2015
The question goes "The rate constant for reaction A \rightarrow B is 25min -1 at 298K and 35min -1 at 350K. Calculate the Value of the rate constant at 770K. Give your anser in min -1 ." How would one approach the question? I tried treating the given numbers as one finds slope, but the ans...
- Sat Feb 06, 2016 2:27 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Question About Variations of the Nernst Equ.
- Replies: 2
- Views: 577
Re: Question About Variations of the Nernst Equ.
Oh, ok. That makes much more sense now. Thank you!
- Fri Feb 05, 2016 8:54 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Question About Variations of the Nernst Equ.
- Replies: 2
- Views: 577
Question About Variations of the Nernst Equ.
So from the course reader, 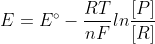 and at 25 degrees Celsius,
and at 25 degrees Celsius, 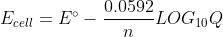
but in the practice midterms, they use this formula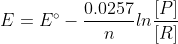 . can someone explain where 0.0257 comes from?
. can someone explain where 0.0257 comes from?
but in the practice midterms, they use this formula
- Fri Jan 29, 2016 4:44 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Reactions
- Replies: 1
- Views: 495
Balancing Redox Reactions
Why must we balance the half reactions if it doesn't affect the standard reduction potential?
- Fri Jan 22, 2016 12:43 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calculating final temperature of a substance
- Replies: 1
- Views: 538
Re: Calculating final temperature of a substance
I'm assuming you're adding the two substances together that then reaches a final temperature. Because the two substances will then share a final temperature, I use the equation q 1 =q 2 therefore mCpT=mCpT Our unknown would then be x, which is the final temperature, not the change in temperature. So...
- Fri Jan 15, 2016 2:20 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Reversible and Irreversible Proccesses
- Replies: 1
- Views: 581
Reversible and Irreversible Proccesses
Can someone explain the difference between a reversible and irreversible process?
If a system at equilibrium is a reversible process, does that mean a system not in equilibrium is irreversible process? But wouldn't systems eventually return to equilibrium?
If a system at equilibrium is a reversible process, does that mean a system not in equilibrium is irreversible process? But wouldn't systems eventually return to equilibrium?
- Wed Jan 06, 2016 5:15 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy
- Replies: 3
- Views: 857
Re: Enthalpy
Enthalpy is a state property because its value is not dependent on the path taken to obtain that state. The best way that I can think of to describe it would be to look at this graph https://upload.wikimedia.org/wikipedia/commons/thumb/2/24/Activation_energy.svg/360px-Activation_energy.svg.png"oncli...
- Fri Dec 04, 2015 3:54 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: SigFigs
- Replies: 2
- Views: 557
Re: SigFigs
Before you take the log of a number, does the number have to equal to the number of Sig figs at the beginning of the problem(what was given) in order for the final value to be correct?
- Wed Nov 18, 2015 6:59 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Question on Oxoacids
- Replies: 1
- Views: 577
Question on Oxoacids
So in the course reader it says that oxoacids lose H+ more readily if the resulting anion can be stabilized by electron withdrawing atoms. I know from lecture that hypochlorous acid is stronger than hypoiodous acid because chlorine is more electron negative compared to iodone, but I dont' understand...
- Wed Nov 11, 2015 9:53 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endo vs. Exothermic
- Replies: 6
- Views: 6412
Re: Endo vs. Exothermic
If heat is applied to an exothermic reaction, the reaction will reach equilibrium by shifting to the left. How Dr. Lavelle explained it during lecture was that if a reaction loses energy to form products(reactants -> products), the reverse reaction would use up energy(products -> reactants). In othe...
- Thu Oct 29, 2015 5:00 pm
- Forum: Hybridization
- Topic: Electron Transfer in Configurations
- Replies: 2
- Views: 659
Re: Electron Transfer in Configurations
I believe we would put the s-orbital electron to the d-orbital when there is about to be 5 or 10 in the d-orbital.
That's why chromium's electron configuration is [Ar] 3d54s instead of [Ar]3d44s2
And copper's electrom configuration is [Ar]3d104s and not [Ar]3d94s2
That's why chromium's electron configuration is [Ar] 3d54s instead of [Ar]3d44s2
And copper's electrom configuration is [Ar]3d104s and not [Ar]3d94s2
- Sat Oct 24, 2015 3:47 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Bond Order and Stability
- Replies: 3
- Views: 1078
Re: Bond Order and Stability
image.jpeg From that example, when writing the electron configurations for O 2 - and N 2 , would we need to specify whether it's 2px, 2py, and 2pz on the quizzes or is that optional? If we do, how to we know what order the 2px/y/z are in or is it just by ascending order? Also, isn't the N 2 MO supp...
- Sat Oct 24, 2015 1:27 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: #5 on the first quiz 2 preparation
- Replies: 3
- Views: 795
Re: #5 on the first quiz 2 preparation
We can describe stability of a molecule with bond order. Bond order can be found by (# of electron in bonding molecular orbital - # of electron in anti-bonding molecular orbitals) divided by two. Anything above 0 means it would be stable, but the bigger the number you get for bond order would mean t...
- Wed Oct 21, 2015 4:09 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pair Repulsion for T-shape
- Replies: 1
- Views: 589
Re: Lone Pair Repulsion for T-shape
From the discussion I had today, if the lone pairs were directly 180 degrees away from each other, that makes all lone pair-central atom-bonded atom angles 90 degrees. Whereas if you had the lone pairs next to each other (120 degrees away from each other), there would be 2 bonded atoms that are 90 d...
- Wed Oct 14, 2015 4:22 pm
- Forum: Ionic & Covalent Bonds
- Topic: Question about Distorted Electrons
- Replies: 2
- Views: 714
Question about Distorted Electrons
Can someone explain to me how distorted electrons work? Is it just the bigger atom drawing the electron from the smaller atom closer to the bigger atom? How is this considered a covalent character?
- Fri Oct 09, 2015 3:57 pm
- Forum: Ionic & Covalent Bonds
- Topic: Equations
- Replies: 3
- Views: 702
Re: Equations
What works for me is I usually see what is given, and what the question is asking for. From there, if the formula used to find what they're looking for doesn't correspond to what was given, I look at what can be found with what was given (ex. given wavelength, I can find frequency).
- Thu Oct 01, 2015 9:18 am
- Forum: Significant Figures
- Topic: All students read this sig fig post [ENDORSED]
- Replies: 170
- Views: 34526
Re: All students read this sig fig post [ENDORSED]
If there is a multistep problem, do we maintain four significant figures per step, even for the following step? For example, if part A requires an answer with only four significant figures (example: 1.178), and part B uses that answer to solve for another variable, should we use 1.178 or the extend...

