Search found 25 matches
- Sun Mar 13, 2016 10:19 am
- Forum: *Cyclohexanes (Chair, Boat, Geometric Isomers)
- Topic: Most Stable Conformation - Larger Substituent
- Replies: 2
- Views: 690
Most Stable Conformation - Larger Substituent
When comparing which substituent is larger to put in the equatorial position of a cyclohexane, do you compare the total mass of the molecule or atomic number of first bonded atom? For example, between Br and ethyl, which one is considered larger to be put in the equatorial position to make the most ...
- Sat Mar 12, 2016 5:30 pm
- Forum: *Identifying Primary, Secondary, Tertiary, Quaternary Carbons, Hydrogens, Nitrogens
- Topic: Tert and Sec Naming
- Replies: 1
- Views: 1330
Tert and Sec Naming
When naming a compound using tert and sec, are we required to put them in parentheses since we cannot it italicize (how it is written in books and online) those terms when writing them? For example, is it 2-tert-butylpentane or 2-(tert)-butylpentane? Or are they both correct and it does not matter?
- Mon Feb 29, 2016 4:19 pm
- Forum: *Cycloalkanes
- Topic: Quiz 3 Preparation 1 Problem #5
- Replies: 1
- Views: 637
Quiz 3 Preparation 1 Problem #5
For the compound for question 5, what is the correct name: 1-bromo-3-iodocyclohexane or 3-bromo-1-iodocyclohexane (book answer)? I have been hearing different reasonings and answers, but I want to know which one is correct and why? When do you give iodo priority with the lower number? And why do you...
- Sun Feb 28, 2016 1:42 pm
- Forum: *Alkanes
- Topic: "Sum Rule"
- Replies: 1
- Views: 513
"Sum Rule"
I still do not quite understand why the "sum rule" does not always work. Can I get a few examples and explanations about why we should not use the "sum rule"?
- Mon Feb 15, 2016 9:52 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate Determining Step
- Replies: 1
- Views: 527
Rate Determining Step
Will the problems always state which step is fast and slow so that we could determine the rate determining step or do we have to some how figure it out based on the context given? If we have to figure it out, how do we know which step is fast and slow?
- Mon Feb 15, 2016 4:57 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Energetically Favorable
- Replies: 1
- Views: 714
Energetically Favorable
When looking at reaction profiles, how do you which reactions are energetically favorable? What does energetically favorable mean?
- Mon Feb 15, 2016 4:54 pm
- Forum: General Rate Laws
- Topic: Rate Law #7 (Quiz 2 Winter 2014)
- Replies: 2
- Views: 718
Re: Rate Law #7 (Quiz 2 Winter 2014)
So you just look for the rate determining step and find the order by looking at the coefficient?
- Mon Feb 15, 2016 4:06 pm
- Forum: General Rate Laws
- Topic: Rate Law #7 (Quiz 2 Winter 2014)
- Replies: 2
- Views: 718
Rate Law #7 (Quiz 2 Winter 2014)
For number 7 the question asks: "The following mechanism was proposed for the reaction: 2NO(g)+O_{2}\rightarrow 2NO_{2}(g) : Step 1(slow): NO+NO\rightarrow N_{2}O_{2} Step 2(fast): O_{2}+N_{2}O_{2}\rightarrow N_{2}O_{4} Step 3(fast): N_{2}O_{4}\rightarrow NO_{2}+NO_{2} What is t...
- Mon Feb 15, 2016 2:49 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Solving for K (Rate Constant)
- Replies: 2
- Views: 750
Solving for K (Rate Constant)
Since there are more than one experiment, which one are you suppose to chose when solving for the rate constant (k)? You get slightly different numbers when using different times and concentrations on the given tables. Is it best to always use the last time and concentration given?
- Tue Feb 09, 2016 9:44 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: E as a Path Function
- Replies: 1
- Views: 561
E as a Path Function
If E is a path function, why is that we are allowed to do  or be able to flip and add the E to calculate the total (similar to Hess's Law)?
or be able to flip and add the E to calculate the total (similar to Hess's Law)?
- Sun Feb 07, 2016 2:32 pm
- Forum: Balancing Redox Reactions
- Topic: Half-Reactions (Midterm 2011 #6)
- Replies: 1
- Views: 555
Half-Reactions (Midterm 2011 #6)
Part A: When looking for the two half-reactions that would create a battery with the largest voltage, do you just look for the two largest positive and negative standard reduction potentials? Part B: After knowing that F_{2}/F^{-} and Rb^{+}/Rb would create the largest voltage, how do you know which...
- Wed Feb 03, 2016 5:33 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Question Regarding Favorable Processes
- Replies: 1
- Views: 506
Gibbs Free Energy Question Regarding Favorable Processes
On page 41 of the course reader where the Gibbs free energy is used to calculate the boiling point, I am confused as to why +\Delta H (endothermic) "does not favor the forward process". How can you determine this? I thought that according to the Le Châtelier's principle, endothermic reacti...
- Mon Jan 25, 2016 11:17 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Question #9 in Quiz 1 Winter 2015
- Replies: 4
- Views: 1107
Re: Question #9 in Quiz 1 Winter 2015
Can someone explain why  and
and  are 0? How do you determine this value?
are 0? How do you determine this value?
- Mon Jan 25, 2016 10:58 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Increase in Entropy (Quiz 1 #7 Winter 2015)
- Replies: 1
- Views: 4220
Increase in Entropy (Quiz 1 #7 Winter 2015)
Question number 7 states: Consider the following processes (treat all gases as ideal). 1) The pressure of 1 mole of oxygen gas is allowed to double isothermally. 2) Carbon dioxide is allowed to expand isothermally to 10 time its original volume. 3) The temperature of 1 mol of helium is increased 25 ...
- Mon Jan 25, 2016 6:13 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Quiz 1 Prep Winter 2014 #9
- Replies: 5
- Views: 1493
Re: Quiz 1 Prep Winter 2014 #9
Dima, are we suppose to multiply 90.83 times 2 because there are 2 moles of HgO in the balanced equation and therefore get deltaH as +181.66? Also for deltaS, I got 216.6 J/Kmol. I did summationS (product) - summationS (reactants): (2(76.02)+205.14) - (2)(70.29). Are these steps correct even though ...
- Sun Jan 24, 2016 11:55 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isobaric (for Solids and Liquids)
- Replies: 1
- Views: 590
Isobaric (for Solids and Liquids)
For reactions with constant pressure that only involve solids and liquids, why and how is 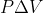 insignificant, which makes
insignificant, which makes 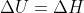 ?
?
- Sat Jan 23, 2016 1:35 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State
- Replies: 1
- Views: 533
Standard State
How do you know if something is in its standard state or form? I am confused as to how to make sure everything is in its standard states. Will questions state that the element is in its standard state/form to know to use the standard reactant enthalpy equation?
- Sun Jan 17, 2016 11:25 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Entropy and Degeneracy
- Replies: 1
- Views: 619
Entropy and Degeneracy
Degeneracy is directly related to entropy, but what does that mean? What is the relationship and difference between entropy and degeneracy? Also, entropy is described as a property, but I am unclear as to what entropy is. Can I please get an explanation?
- Sat Jan 09, 2016 9:51 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive and Intensive Properties
- Replies: 1
- Views: 636
Extensive and Intensive Properties
In the course reader it states that, "Heat capacity is an extensive property" and that "Specific heat capacity is an intensive property" (pages 5-6). What is an extensive property and an intensive property? I remembered from lecture that an extensive property is something like de...
- Tue Nov 24, 2015 3:57 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Video: Quantum Numbers
- Replies: 1
- Views: 560
Video: Quantum Numbers
Overview of Quantum Numbers
- Mon Oct 26, 2015 12:15 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Bond Order
- Replies: 1
- Views: 498
Bond Order
What are bond orders and how do you determine them? How can bond orders equal .5?
- Sun Oct 25, 2015 11:57 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: H2CBr2 Polar and Dipole Moments
- Replies: 1
- Views: 845
H2CBr2 Polar and Dipole Moments
Is 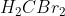 polar? If so, why is it polar because it seems as if the arrangement of the atoms could be symmetrical?
polar? If so, why is it polar because it seems as if the arrangement of the atoms could be symmetrical?
Also, can you explain how dipole moments cancel?
Also, can you explain how dipole moments cancel?
- Wed Oct 21, 2015 9:56 am
- Forum: Ionic & Covalent Bonds
- Topic: Electron Configurations of Copper (I) and Copper (II)
- Replies: 2
- Views: 15506
Electron Configurations of Copper (I) and Copper (II)
What are the electron configurations of:
(a) the copper(I) ion
(b) the copper(II) ion
(c) the manganese(II) ion
(d) the lead(IV) ion
What do the (I), (II), and (IV) mean and how do they change the electron configurations?
(a) the copper(I) ion
(b) the copper(II) ion
(c) the manganese(II) ion
(d) the lead(IV) ion
What do the (I), (II), and (IV) mean and how do they change the electron configurations?
- Sat Oct 10, 2015 9:28 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589305
Re: Chemistry Jokes
Q: Where does a chemist put the dishes?
A: In the Zinc, of course!
Q: What did the thermometer say to the graduated cylinder?
A: "You may have graduated but I have many degrees!"
Q: Does light have mass?
A: Of course not! It's not even Catholic!!!
A: In the Zinc, of course!
Q: What did the thermometer say to the graduated cylinder?
A: "You may have graduated but I have many degrees!"
Q: Does light have mass?
A: Of course not! It's not even Catholic!!!
- Sun Oct 04, 2015 5:52 pm
- Forum: Einstein Equation
- Topic: Understanding Planck's Constant
- Replies: 1
- Views: 700
Understanding Planck's Constant
I know that Planck's Constant is 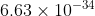 J.s but how was this number derived and why is it used as "h" for the E=hv equation? What exactly is Planck's Constant?
J.s but how was this number derived and why is it used as "h" for the E=hv equation? What exactly is Planck's Constant?

