Search found 24 matches
- Sat Mar 12, 2016 12:41 pm
- Forum: *Cycloalkanes
- Topic: Question 9A on Winter 2015 Final
- Replies: 1
- Views: 568
Re: Question 9A on Winter 2015 Final
Because the parent chain always has to include the functional group. In this case, the cycleheptane ring doesn't have the alcohol group directed attached to it, so you have to consider the smaller propane chain to be the parent instead.
- Fri Mar 11, 2016 12:01 am
- Forum: *Cyclohexanes (Chair, Boat, Geometric Isomers)
- Topic: Determining Steric/Torsional Interactions
- Replies: 3
- Views: 909
Re: Determining Steric/Torsional Interactions
Torsional strain is the strain that exists when atoms are eclipsed with respect to each other. Steric strain, on the other hand, exists when atoms are physically in contact with one another (e.g. flagpole interaction in the boat conformation).
- Tue Mar 08, 2016 1:50 am
- Forum: Administrative Questions and Class Announcements
- Topic: Question about the final
- Replies: 1
- Views: 599
Re: Question about the final
Yes, Dr. Lavelle mentioned that the questions will be longer than they were on the midterm, but that we should still have more than enough time within the almost 3 hours.
- Wed Mar 02, 2016 1:55 am
- Forum: *Alkynes
- Topic: Parentheses confusion
- Replies: 2
- Views: 1688
Re: Parentheses confusion
If, for example, you have the formula CH3CH2CH(CH3)C(CH3)3, you would be able to see clearly the the CH3 in parenthesis in the middle of the formula is a substituent, since a single carbon can't have four bonds (3 H's and 2 of the C's right next to it). Also, you would always include ONE of the mole...
- Thu Feb 25, 2016 7:08 pm
- Forum: *Electrophiles
- Topic: Memory Tips
- Replies: 8
- Views: 1854
Re: Memory Tips
You can also think of nucleophiles as being "negative" because both words start with the letter N; thus, having a negative charge would cause the nucleophile to be electron-rich. All you have to do from there is just remember that electrophiles are the opposite of nucleophiles!
- Sat Feb 20, 2016 11:35 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: reaction order
- Replies: 3
- Views: 769
Re: reaction order
You can use the general formula for a rate constant's units to determine this: (M^-(n-1))(s^-1), where n is the order of the reaction. So, starting with a zero-order reaction, the units of k would be M/s. You then just have to divide by M each time you "increase" the order by one.
- Thu Feb 18, 2016 12:10 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Quiz 2 prep #1
- Replies: 3
- Views: 773
Re: Quiz 2 prep #1
Yes, A is the reactant and P is the product.
- Sun Feb 14, 2016 9:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to add Pt(s)
- Replies: 5
- Views: 8765
Re: When to add Pt(s)
You only add Platinum to the cell diagram/redox reaction when there is no solid already in the anode or in the cathode of the cell. In this case, you would just add Pt (s) to whichever portion does not already have a solid. If both the anode and cathode are without solids, then you would add Pt (s) ...
- Sat Feb 06, 2016 12:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cathodes and anodes
- Replies: 1
- Views: 461
Re: cathodes and anodes
For the most part, the cathode should be on the right and the anode on the left. However, if the question specifies that it is the opposite, you should just remember to do all of your calculations with the specified orientation in mind. Basically, if it doesn't specify, assume that the cathode is on...
- Thu Jan 28, 2016 2:01 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Reversible vs. Irreversible
- Replies: 1
- Views: 418
Re: Reversible vs. Irreversible
I think that in regards to entropy calculations, the question itself should tell you that an isothermal, reversible process is occurring. For reversible reactions then, use the equation 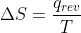 .
.
- Thu Jan 21, 2016 9:48 pm
- Forum: Calculating Work of Expansion
- Topic: Specific heat capacity and molar heat capacity
- Replies: 3
- Views: 3606
Re: Specific heat capacity and molar heat capacity
Specific heat capacity and molar heat capacity are essentially the same concept -- the only different is that specific heat capacity (Csp) deals with mass in terms of grams while molar heat capacity (Cm) deals with mass in terms of moles. Heat capacity, however, does not account for the substance's ...
- Thu Jan 14, 2016 5:26 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Affecting Isolated Systems
- Replies: 2
- Views: 623
Re: Affecting Isolated Systems
I also believe that there is no way to change the energy of an isolated system -- since you cannot heal or cool such a system, or even compress or expand its volume through work, there seems to be no way to actually alter its internal energy.
- Fri Jan 08, 2016 9:08 pm
- Forum: Phase Changes & Related Calculations
- Topic: Dividing Heat Capacity
- Replies: 2
- Views: 545
Re: Dividing Heat Capacity
From what I understood from lecture, heat capacity in its intensive form is more useful because it allows us to know the actual, specific amount of substance being dealt with. As an extensive property, however, we are not sure how "large" the object of interest actually is, and so our over...
- Mon Nov 30, 2015 2:51 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Exceptions of anions
- Replies: 2
- Views: 726
Re: Exceptions of anions
Because they are both derived from strong polyprotic acids (H2SO4 and H2PO4), meaning that their parent acids can donate more than one proton. As a result, HSO4- and H2PO4- are considered weak acids in contrast to their strong parent acids, but can still donate a proton even though it will be much h...
- Wed Nov 25, 2015 1:45 am
- Forum: Amphoteric Compounds
- Topic: Location of amphoteric compounds in periodic table?
- Replies: 2
- Views: 1083
Re: Location of amphoteric compounds in periodic table?
For the most part, non-metal oxides will always be acidic and metal oxides (particularly the transition metals) will always be basic. So whatever is left over (i.e. the metalloids: Be, Si, Ge, As, Sb, Te, Po) exhibits both acidic and basic properties and thus forms amphoteric compounds.
- Thu Nov 19, 2015 2:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Volume and Concentration
- Replies: 2
- Views: 625
Re: Volume and Concentration
If you decrease the volume, the pressure increases (from the inverse relationship between pressure and volume in the ideal gas law: PV=nRT). So if you decrease the volume, the side with more moles (let's say the products, for this example) will have its volume more significantly decreased causing th...
- Thu Nov 12, 2015 12:04 pm
- Forum: Hybridization
- Topic: D orbital vs S orbital
- Replies: 2
- Views: 632
Re: D orbital vs S orbital
In its ground state, the electrons in Chromium's 4s orbital are at a higher energy level, and thus are less stable than the electrons in the 3d orbitals which are at a lower energy level. When electrons are being removed, it is always easier to remove electrons that are at a higher energy level; as ...
- Wed Nov 04, 2015 12:19 am
- Forum: Dipole Moments
- Topic: Polarity of Double Bonds
- Replies: 1
- Views: 3550
Re: Polarity of Double Bonds
No, for our purposes, as long as the molecule is symmetrical with no lone pairs on the center atom (as in this case, where the molecular shape is tetrahedral and the P has no lone pairs, causing the dipole moments between the P and each O to cancel each other out) the molecule is non-polar.
- Mon Oct 26, 2015 9:10 pm
- Forum: Ionic & Covalent Bonds
- Topic: Cations and Anions [ENDORSED]
- Replies: 12
- Views: 2182
Re: Cations and Anions [ENDORSED]
A cation is an ion or group of ions (an atom or molecule) with a total positive charge, while an anion is one with a total negative charge.
- Tue Oct 20, 2015 9:40 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Spins
- Replies: 3
- Views: 811
Re: Electron Spins
The point of assigning spin states is to distinguish between the two electrons that can fill each orbital; the +1/2 and -1/2 ms values are essentially arbitrary, as they do not actually describe a physical phenomenon experienced by the electrons but simply serve to clarify which electron is which wh...
- Wed Oct 14, 2015 2:03 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Spin State
- Replies: 3
- Views: 748
Re: Spin State
Yes, the spin state orientation for a particular electron in an orbital is arbitrary. However, when you continue to fill the subshell with electrons, placing one electron in each subsequent orbital at a time, these electrons must have the same spin state as the first one's. In accordance with Hund's...
- Tue Oct 06, 2015 1:48 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Not understanding the use of ml
- Replies: 2
- Views: 689
Re: Not understanding the use of ml
From what I understand, ml refers to the magnetic quantum number and determines the orientation of the orbital in question by labeling the different orbitals of a subshell with specific numerical values. Since ml can take the any value within the range of -l to l, each of those values would correspo...
- Tue Sep 29, 2015 4:40 pm
- Forum: Empirical & Molecular Formulas
- Topic: Molecular Formulas
- Replies: 3
- Views: 1053
Re: Molecular Formulas
If the problem directly tells you that the substance consists of 15 g of C, 2 g of H, and 3 g of O, and that the total mass of the substance is 20 g, then yes, you would just directly calculate the appropriate percentage compositions by dividing 15 g by 20 g for the percent composition of C, 2 g by ...
- Mon Sep 28, 2015 10:33 pm
- Forum: Empirical & Molecular Formulas
- Topic: How to find the empirical formula being given only masses?
- Replies: 3
- Views: 949
Re: How to find the empirical formula being given only masse
Hi Elizabeth! As far as I know, the way you solve this problem is by initially separating your calculations into two steps. You first find the grams of carbon (C) and hydrogen (H) present in the compound in the first chemical reaction of: compound + O2 --> CO2 + H2O by using the apparent molar ratio...

