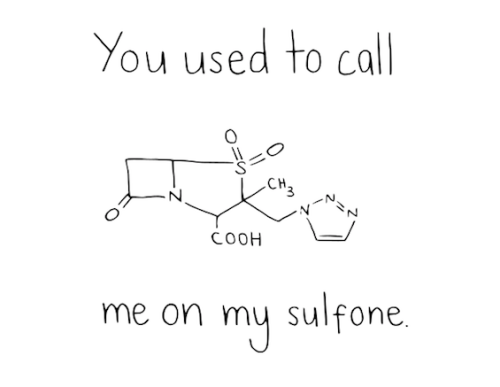
Hope this question makes sense.. These are two examples taken from Problem Set 2
(E )-3-bromo-2,4-dimethylhepta-2,4-diene
(Z)-4-ethyl-6-methylhept-3-enal
Why is one hepta- and one hept-? What are the rules when it comes to adding/dropping the -a?
Search found 22 matches
- Sat Mar 18, 2017 2:27 am
- Forum: *Cycloalkanes
- Topic: Root naming
- Replies: 2
- Views: 1412
- Fri Mar 17, 2017 10:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Winter 2016 Final Exam Q2A [ENDORSED]
- Replies: 7
- Views: 1506
Re: Winter 2016 Final Exam Q2A [ENDORSED]
Kind of unrelated question but: why is it 2X on the top? I didn't quite get that in the review session. Do you mind explaining that part? The reactants are half of the products aka the products are double the reactants. He made the concentration of the reactants "x," so products would be ...
- Tue Mar 14, 2017 4:20 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
- Mon Mar 06, 2017 9:28 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Quiz 3 Winter 2017
- Replies: 183
- Views: 29651
Re: Quiz 3 Winter 2017
Leo_Navejas_3B wrote:For #4 Why isn't it 1-butyl-3,4 dimethylcyclohexane?
Page 96 in the course reader should help.
- Sun Mar 05, 2017 1:36 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Quiz 3 Winter 2017
- Replies: 183
- Views: 29651
Re: Quiz 3 Winter 2017
For number 7, are we expected to know what 2-butene is?
- Sat Mar 04, 2017 11:53 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
- Sat Feb 25, 2017 2:37 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
- Sun Feb 19, 2017 10:02 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
did you know the sun doesn't have to go to college cause it already has 28 million degrees :-)
- Tue Feb 14, 2017 3:06 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Question 8, Winter 2013 Midterm
- Replies: 1
- Views: 508
Re: Question 8, Winter 2013 Midterm
I think we're going to be given a chart with various reduction potentials
- Fri Feb 10, 2017 12:31 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
What do you call an acid with an attitude...
A mean o' acid
A mean o' acid
- Fri Feb 03, 2017 1:05 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
What did Cesium and Iodine do Saturday Night?
Watch CsI.
Watch CsI.
- Sat Jan 28, 2017 11:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: General Explanation of sig figs
- Replies: 1
- Views: 522
Re: General Explanation of sig figs
1. Zeros don't count... (i.e 400 has one sigfig) 2. UNLESS it is after a decimal (i.e 4.0 has two sigfigs and 40.0 has three) General rule of thumb is to not round anything until your final calculation aka the answer. Once you get the answer, you take the count of the number that has the least sigfi...
- Sat Jan 21, 2017 8:24 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
What did the chemist say when he found two helium atoms?
HeHe
HeHe
- Fri Jan 13, 2017 7:51 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
Did you hear about the man who got cooled to absolute zero?
....
He's 0K now.
....
He's 0K now.
- Fri Nov 25, 2016 12:41 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
I told a joke about helium but no one reacted..
- Fri Nov 18, 2016 10:21 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
- Thu Nov 10, 2016 5:04 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Heteronuclear diatomic molecular orbital
- Replies: 2
- Views: 554
Re: Heteronuclear diatomic molecular orbital
Probably not as formal of an answer you're looking for, but basically it has to do with the idea that "you're only as strong as your weakest link." We can't use the Z>8 diagram if one of the elements in the bond does not have an atomic number more than 8. Whereas Z<8 both are applicable be...
- Fri Nov 04, 2016 10:04 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
- Wed Oct 26, 2016 10:30 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589377
Re: Post All Chemistry Jokes Here
Why does hamburger yield lower energy than steak?
Because it's in the ground state.
Because it's in the ground state.
- Fri Oct 21, 2016 12:07 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge Meaning
- Replies: 2
- Views: 1132
Re: Formal Charge Meaning
Formal charge is the difference between the number of valence electrons of each atom and the number of electrons the atom is associated with. Formal charge assumes any shared electrons are equally shared between the two bonded atoms.
- Sat Oct 08, 2016 7:58 pm
- Forum: SI Units, Unit Conversions
- Topic: Quiz Prep: Conversions [ENDORSED]
- Replies: 2
- Views: 850
Re: Quiz Prep: Conversions [ENDORSED]
Yes, Lavelle told our lecture that we will need to know all the SI units. My TA said that we should know from as big as giga- and little as pico-.
- Fri Sep 30, 2016 5:32 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric effect
- Replies: 2
- Views: 541
Re: Photoelectric effect
Yes, electrons will be emitted as long as the threshold energy is met. If the amount of energy in the photon is equal to the threshold energy, there is just no kinetic energy.