Search found 13 matches
- Sat Mar 18, 2017 7:59 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 570963
Re: Saying Thank You to Dr. Lavelle
Thank you Dr. Lavelle and Dr. Lavelle's amazing course reader. :^)
- Sat Mar 18, 2017 7:55 pm
- Forum: *Calculations Using ΔG° = -RT ln K
- Topic: How are the questions using ΔG°=-RT ln k phrased?
- Replies: 3
- Views: 1591
How are the questions using ΔG°=-RT ln k phrased?
For the calculations comparing the ratio of two conformations using ΔG° = -RT ln K, is that going to be in the final?
If it is, how are the questions usually phrased?
If it is, how are the questions usually phrased?
- Mon Mar 13, 2017 10:08 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Final Exam 2013 Question 1B
- Replies: 1
- Views: 479
- Fri Mar 10, 2017 7:58 pm
- Forum: *Cycloalkanes
- Topic: Constitutional Isomers
- Replies: 2
- Views: 1067
Constitutional Isomers
Hi,
I still don't understand how to come up with constitutional isomers. For example, when they ask for the cycloalkane isomers of C8H16, how do I figure it out?
I still don't understand how to come up with constitutional isomers. For example, when they ask for the cycloalkane isomers of C8H16, how do I figure it out?
- Tue Mar 07, 2017 11:34 pm
- Forum: *Nucleophiles
- Topic: Ambident
- Replies: 3
- Views: 1439
Re: Ambident
An ambident nucleophile is a molecule that has more than one nucleophilic site and has a negative charge delocalized by resonance. To identify the ambident structure, you would need to know which molecules have resonance structures. If you are not able to determine from the chemical equation which o...
- Tue Mar 07, 2017 9:34 pm
- Forum: *Electrophilic Addition
- Topic: Steric Contriubtion to Standard Enthalpies of Activation
- Replies: 1
- Views: 1370
Re: Steric Contriubtion to Standard Enthalpies of Activation
We learn that \Delta S^{\circ}^{\ddagger } values are often unfavorable, negative values because there is usually a decrease in entropy when reactants go into the transition state. Going into the transition state, there are specific orientation requirements for the reaction and these specifications ...
- Thu Feb 23, 2017 3:16 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate Law
- Replies: 2
- Views: 614
Re: Rate Law
Yes, to determine the rate determining step the slowest of the multiple steps is used to dictate the overall reaction rate. For a proposed mechanism to be valid, the following conditions MUST be satisfied (no exceptions): 1. The elementary steps sum to the overall reaction. 2. The rate law of the sl...
- Thu Feb 23, 2017 2:48 pm
- Forum: First Order Reactions
- Topic: Deriving the 1st order reaction equation
- Replies: 2
- Views: 534
Re: Deriving the 1st order reaction equation
Deriving the First Order Reaction Rate = k[A] Rate = -\frac{d[A]}{dt} \therefore -\frac{d[A]}{dt} = k[A]^{1} \frac{d[A]}{dt} = -k[A]^{1} \frac{d[A]}{[A]} = -kdt \frac{1}{[A]}d[A] = -kdt \int_{[A]_{0}}^{[A]_{t}} \frac{1}{[A]} d[A] = - \int_{0}^{t} kdt \int_{[A]_{0}}^{[A]_{t}} \frac{1}{[A]} d[A] = -k...
- Fri Feb 10, 2017 5:16 pm
- Forum: Balancing Redox Reactions
- Topic: Self Test 14.2 B
- Replies: 2
- Views: 573
Re: Self Test 14.2 B
The equation for the reaction of iodide with iodate should be:
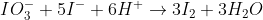
Does that make it easier to figure out the oxidation/reduction part?
Does that make it easier to figure out the oxidation/reduction part?
- Fri Feb 10, 2017 5:06 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 14.15 (a)
- Replies: 1
- Views: 474
Re: Homework 14.15 (a)
If you look in the back of the book at Appendix 2B, you can find the half reactions (remember that these are reduction half reactions, though). Ag^+ + e^- \rightarrow Ag AgBr + e^-\rightarrow Ag + Br^- You are unable to use Br_{2} + 2e^- \rightarrow 2Br^- because there is no Br_{2} in the equation. ...
- Thu Feb 02, 2017 4:24 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: When does ΔG = 0?
- Replies: 1
- Views: 405
When does ΔG = 0?
I know that it equals to 0 at equilibrium, but what are some examples of it being in equilibrium?
- Fri Jan 27, 2017 1:10 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Cv and Cp
- Replies: 1
- Views: 491
Re: Cv and Cp
Yeah I think it'll be best to memorize them or at least know how to deduce them because Dr. Lavelle had done a few calculations with them in lecture. In Chapter 9, there were some problems that required this concept and just make sure you can distinguish the difference between when to use atoms, lin...
- Sat Jan 21, 2017 5:51 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: definition of isothermic reversible expansion [ENDORSED]
- Replies: 1
- Views: 629
Re: definition of isothermic reversible expansion [ENDORSED]
An isothermal reversible expansion is basically just a process conducted where the temperature remains constant during the entire operation. So in an isothermal reversible expansion, you have a gas cylinder with a piston. The piston is placed in a constant temperature setting (like a water bath; thi...

