Search found 21 matches
- Fri Mar 17, 2017 4:42 pm
- Forum: *Cycloalkanes
- Topic: 2015 Practice Final 9A
- Replies: 1
- Views: 1343
Re: 2015 Practice Final 9A
In this case I think the cycloheptane is treated as a substituent while the propane is treated as the main carbon chain because propane contains the functional OH group, and the main/longest carbon chain must always contain the functional group. This explains the anomalous naming.
- Tue Feb 28, 2017 8:12 pm
- Forum: *Alkanes
- Topic: Multiple Line Structures
- Replies: 1
- Views: 448
Multiple Line Structures
When drawing line structures, what makes one line structure more valid/correct than another? For example... https://snag.gy/ZQDNoi.jpg All represent 3,3-dimethylpentane, yet each differ slightly by bond angle or whether a line turns up or down. Is there a standardized correct method for these line s...
- Tue Feb 28, 2017 1:22 am
- Forum: *Alkanes
- Topic: prop-1-enyl vs prop-1-ene
- Replies: 1
- Views: 557
prop-1-enyl vs prop-1-ene
I've seen both used in the textbook. Is prop-1-enyl simply a way of writing prop-1-ene when a substituent of Benzene? They both seem to refer to a propyl with a double bond on the 1 to 2 carbon.
- Mon Feb 13, 2017 3:19 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Sig Figs for K Calculations from Gibbs Free Energy
- Replies: 1
- Views: 402
Re: Sig Figs for K Calculations from Gibbs Free Energy
I understand that the least number of given sig figs is the number of sig figs you use, but does that mean in this case we just ignore all log rules we learned for pH? And which case there's still the question of if we use the rounded value of G or the unrounded value for additional precision (the a...
- Mon Feb 13, 2017 3:15 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Sig Figs for K Calculations from Gibbs Free Energy
- Replies: 1
- Views: 402
Sig Figs for K Calculations from Gibbs Free Energy
For example (#6 in Midterm 2015) we must calculate E^{\circ} , \Delta G ^{\circ} , K . With a calculated E^{\circ} = 0.82V , we get that \Delta G ^{\circ} = -nFE^{\circ} = -1.582... \times 10^5 \approx -1.6 \times 10^5 J . When we proceed to calculate K from \Delta G^{\circ} = -RTlnK , do we use the...
- Thu Feb 02, 2017 7:12 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: How to keep track of significant figures?
- Replies: 2
- Views: 837
How to keep track of significant figures?
For example in HW Question 14.39 using the Nernst equation to solve for the concentration of a compound in a battery, how many significant figures does the final answer have? You have to keep track of the ln rules, and then there's the fact that you're subtracting one term from another, so you have ...
- Wed Feb 01, 2017 12:54 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: HOMEWORK 9.35
- Replies: 2
- Views: 575
Re: HOMEWORK 9.35
A single atom can move only translationally in 3 axises, so 3\times \frac{1}{2}RT = \frac{3}{2}RT . A nonlinear molecule can move translationally, but also rotate along 3 axises, so \frac{3}{2}RT + 3(\frac{1}{2}RT) = \frac{6}{2}RT = 3RT . A linear molecule would be similar but it can only ro...
- Tue Jan 24, 2017 5:50 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Significant figures for Boltzmann Equation
- Replies: 1
- Views: 386
Significant figures for Boltzmann Equation
I know that for logs, the rule is the log(X) where X has n significant figures means the answer must go out to n decimal places. However, this property only made sense in the context of 10^x, where the base = 10. What is the rule for ln, where the base is ~2.72, is it the same as logs?
- Tue Jan 24, 2017 5:39 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: What is "standard" [ENDORSED]
- Replies: 1
- Views: 406
What is "standard" [ENDORSED]
What is the exact definition of Standard Enthalpy, entropy, and gibbs free energy? Is it at a defined temperature and pressure? What is a "pure state" for gibbs free energy standard.
- Tue Jan 17, 2017 1:31 pm
- Forum: Phase Changes & Related Calculations
- Topic: How can internal energy be zero when isothermal reversible expansion?
- Replies: 1
- Views: 551
How can internal energy be zero when isothermal reversible expansion?
Why is 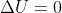 when a gas expands reversibly and isothermally? Because
when a gas expands reversibly and isothermally? Because 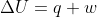 and w < 0 (work being done on surroundings), where does the +q come from to counteract internal energy leaving as work?
and w < 0 (work being done on surroundings), where does the +q come from to counteract internal energy leaving as work?
- Wed Jan 11, 2017 8:52 pm
- Forum: Phase Changes & Related Calculations
- Topic: Reversible Isothermal Reactions
- Replies: 1
- Views: 482
Reversible Isothermal Reactions
Why does a reversible reaction need to be isothermal for us to calculate the work done? How does work relate to temperature?
Why does U = 0 when isothermal compression/expansion of gas?
Why does U = 0 when isothermal compression/expansion of gas?
- Sun Dec 04, 2016 7:35 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 4018764
- Mon Nov 14, 2016 6:57 pm
- Forum: Naming
- Topic: Rules for Writing Formula [ENDORSED]
- Replies: 1
- Views: 633
Rules for Writing Formula [ENDORSED]
What are the rules for writing the formula of coordination compounds? Is it alphabetical like writing the name? And if so, is it alphabetical by the symbol (Na = N) or the actual name (Sodium = S). I've also heard that neutral ligands are written before charged ones, is this true? Does charge affect...
- Sat Nov 05, 2016 4:32 pm
- Forum: Naming
- Topic: HW 17.31 About alphabetic sequence in writing symbols and naming them
- Replies: 1
- Views: 383
Re: HW 17.31 About alphabetic sequence in writing symbols and naming them
1) I think writing it as (OH2) is safer because that's how the book does it.
2) In naming you write the neutral ligands first (so OH2 first because it's neutral). Otherwise it's alphabetical, and if 2 ligands contain the same element the ligand with fewer elements comes first.
2) In naming you write the neutral ligands first (so OH2 first because it's neutral). Otherwise it's alphabetical, and if 2 ligands contain the same element the ligand with fewer elements comes first.
- Thu Nov 03, 2016 11:29 am
- Forum: Lewis Structures
- Topic: Best Method for Drawing Lewis Structures
- Replies: 1
- Views: 522
Best Method for Drawing Lewis Structures
My TA has a great way of doing Lewis Structures, evaluating the valence electrons of each atom one by one instead of totaling up the electrons as a whole and arbitrarily filling octets. I feel like this way better shows how the actual bonds form; for example in CO2 in doing it this way you see each ...
- Tue Oct 25, 2016 8:29 pm
- Forum: Lewis Structures
- Topic: N2O Lewis Structure; how can N form a double bond?
- Replies: 1
- Views: 12156
N2O Lewis Structure; how can N form a double bond?
https://upload.wikimedia.org/wikipedia/commons/3/33/Muatan_masing-masing_atom_pada_N2O.jpg In structure 2, how can the N form a double bond (2 electrons contributed) and have 2 lone pair of elections (4 electrons). 2e- + 4e- = 6e-, but nitrogen only has 5 valence electrons. How come?
- Mon Oct 17, 2016 1:59 pm
- Forum: Lewis Structures
- Topic: Best Way to Determine Central Atom [ENDORSED]
- Replies: 1
- Views: 1769
Best Way to Determine Central Atom [ENDORSED]
The textbook says least electronegative while Doctor Lavelle says lowest ionization energy or electron affinity.
What is the difference between electron affinity and electronegativity? Which way is most reliable to determine central atom?
What is the difference between electron affinity and electronegativity? Which way is most reliable to determine central atom?
- Mon Oct 10, 2016 1:05 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Aufbau Principal Exceptions
- Replies: 1
- Views: 538
Aufbau Principal Exceptions
Can someone explain why Ag's electron configuration is abnormal? What other exceptions should we know?
- Mon Oct 10, 2016 1:03 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity
- Replies: 3
- Views: 1000
Electron Affinity
The textbook says electron affinity increases to the right. But what about up and down? Whats the explanation for the trend?
- Mon Oct 10, 2016 1:01 pm
- Forum: *Shrodinger Equation
- Topic: Shrodinger Equation
- Replies: 4
- Views: 928
Shrodinger Equation
So I know that we dont actually have to know how to do calculations with the equation. So what DO we need to know about the equation conceptually?
- Fri Sep 30, 2016 12:58 am
- Forum: Properties of Light
- Topic: Mass of Atoms to Energy
- Replies: 1
- Views: 1281
Re: Mass of Atoms to Energy
You have to use DeBroglie's equation, pictured below.
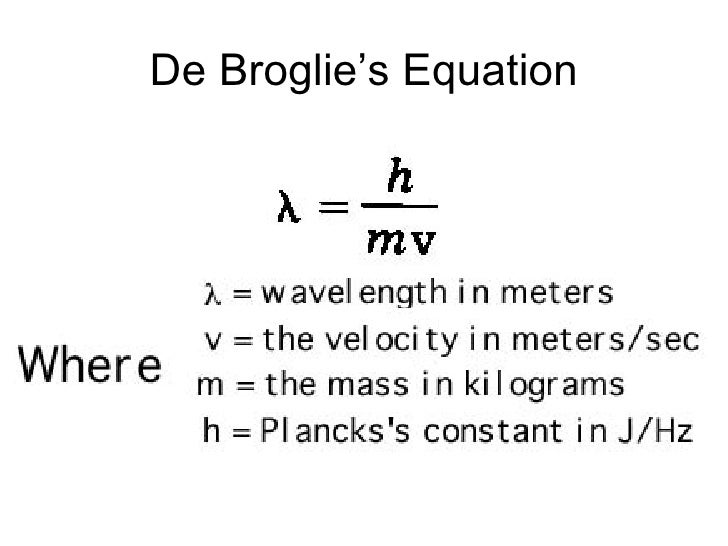
From there you can find the frequency from the wavelength and the energy from E = hv, or alternatively e = hc/λ.

From there you can find the frequency from the wavelength and the energy from E = hv, or alternatively e = hc/λ.


