I would highly doubt it. I believe that functional groups that are listed in the course reader pages 97-98 (and how to name them on page 99) are all that we'd need to know, even if the Intro to Organic Chemistry book might talk about other functional groups.
If I'm wrong, please correct me!
Search found 35 matches
- Sun Mar 12, 2017 8:20 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation
- Replies: 1
- Views: 484
Re: Arrhenius Equation
Usually no. You can probably determine it if they give you everything else in the equation (rate constant k, activation energy, temperature), but usually it's not necessary to know A to solve a problem (if ever).
- Tue Feb 28, 2017 4:31 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Quiz 3 & Extra Credit
- Replies: 2
- Views: 662
Re: Quiz 3 & Extra Credit
The way I understood it, the extra credit is so that your lowest quiz score will be replaced with your highest quiz score, so yes in a way, your highest score will be recorded twice, and the lowest score will be dropped. I can only speculate, but I would think it will include the rest of kinetics an...
- Sun Feb 26, 2017 11:02 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation Energy
- Replies: 3
- Views: 1258
Re: Activation Energy
I don't believe you can tell based on (solely) activation energy if a reaction is exothermic or endothermic. Looking at whether the reactants have more energy than the products should be a better way of figuring out whether or not a reaction is exothermic or endothermic. If the reaction is endotherm...
- Tue Feb 21, 2017 5:04 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591247
Re: Post All Chemistry Jokes Here
I'm feeling STOICHED for chem.
- Sun Feb 12, 2017 11:56 am
- Forum: Balancing Redox Reactions
- Topic: Balancing Reactions
- Replies: 1
- Views: 511
Re: Balancing Reactions
Yes, this would generally be the correct procedure. https://chem.libretexts.org/Core/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Balancing_Redox_reactions This page goes step by step with neutral, acidic, and basic solutions. You'll probably have to be careful with adding the OH- at the en...
- Thu Feb 02, 2017 7:36 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Trouton's rule
- Replies: 4
- Views: 1364
Re: Trouton's rule
No, it shouldn't be required. I didn't see a problem on my quiz that was about Trouton's Rule.
- Wed Feb 01, 2017 7:41 pm
- Forum: Calculating Work of Expansion
- Topic: P delta(V) =delta(n)RT
- Replies: 2
- Views: 1197
Re: P delta(V) =delta(n)RT
I suppose you could use it any time you think you need to use P \Delta V but you don't have P and/or V. Some homework problems have it so that you would have to solve for work, but they don't give you P or \Delta V. Oftentimes, these homework problems will give you the mols of the element/compound i...
- Sun Jan 29, 2017 4:10 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 2
- Views: 608
Re: Gibbs Free Energy
Also, Gibbs free energy isn't something that we can directly measure. It's defined by other measurements that happen to be state functions (if you take a look at the equation, it uses things like enthalpy and entropy, which are state functions). So, I suppose it just ends up being a state function.
- Sun Jan 22, 2017 3:21 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Example 8.13 in Textbook
- Replies: 2
- Views: 664
Re: Example 8.13 in Textbook
I believe what they're trying to say is that by breaking the Br-Br bonds, the Br molecules were free to bond with the C's, and thus there are 2 C-Br bonds that were created in the products. CH3CH=CH2 has 1 C-C bond and 1 C=C bond, and in order for the C-Br bonds to be formed, the C=C bond was broken...
- Mon Jan 16, 2017 11:10 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Enthalpy [ENDORSED]
- Replies: 3
- Views: 746
Re: Enthalpy [ENDORSED]
Enthalpy is the amount of heat released or absorbed at a constant pressure. For a single bond in a compound to become a double bond would require the compound's single bond to be broken, and then a double bond to be formed. Breaking bonds require energy, while forming bonds would release energy. Bre...
- Fri Dec 02, 2016 10:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: ax2e2 question [ENDORSED]
- Replies: 1
- Views: 532
Re: ax2e2 question [ENDORSED]
It would be bent. An example of AX2E2 would be H2O; O is the central atom A and has 2 lone pairs (E2), and the two H's are X2.
- Thu Nov 17, 2016 12:50 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: polydentate ligands [ENDORSED]
- Replies: 2
- Views: 1160
Re: polydentate ligands [ENDORSED]
Basically if that ligand has multiple places it can bond. Ligands like NH 3 (ammine) and (OH) - (hydroxo) can only bond in one place (for ammine, it bonds by the N, and hydroxo can bond by O). So, those ligands would be monodentates. Ethylenediamine (en) can bond in two places (there are 2 N's that ...
- Mon Nov 07, 2016 7:47 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: MO theory
- Replies: 3
- Views: 750
Re: MO theory
Higher bond orders generally mean more stability, yes, and whatever you calculate to be your bond order generally seems to correspond to a type of bond (meaning, 0=no bond, 1=single bond, 2=double bond, 3=triple bond, though you can have bond orders of 1.5, 2.5, etc, so you can see why higher bond o...
- Mon Oct 24, 2016 2:06 pm
- Forum: Ionic & Covalent Bonds
- Topic: Stronger bonds
- Replies: 3
- Views: 1897
Re: Stronger bonds
There are covalent bonds and ionic bonds. Covalent bonds "share" their electrons between them, while in ionic bonds, one of the atoms "takes" the electrons of the other atom to complete its electron shell. The greater the difference is between the two atom's electronegativity val...
- Mon Oct 17, 2016 3:21 pm
- Forum: Lewis Structures
- Topic: Best Way to Determine Central Atom [ENDORSED]
- Replies: 1
- Views: 1767
Re: Best Way to Determine Central Atom [ENDORSED]
The trend for electronegativity, electron affinity, and ionization energy is the same on the periodic table. As you go up and to the right, electronegativity, electron affinity, and ionization energy increases. So saying least electronegative is similar to saying lowest ionization energy or lower e...
- Mon Oct 17, 2016 2:52 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals [ENDORSED]
- Replies: 9
- Views: 1849
Re: Orbitals [ENDORSED]
There's no just "m" quantum number, there is m l and m s . The m l describes the different orbitals in the subshell (There's the subshell p, and m l would show differentiate between p x , p y , and p z orientations). m s on the other hand tells you if one of the electrons (in one orientati...
- Mon Oct 17, 2016 2:32 pm
- Forum: General Science Questions
- Topic: Determining reactants given the products [ENDORSED]
- Replies: 1
- Views: 942
Re: Determining reactants given the products [ENDORSED]
I suppose it depends. There are different types of reactions, such as synthesis, decomposition, single replacement, double replacement, etc. Synthesis: If you're only given a compound, like NaCl, you can guess that the reactants were probably Na and Cl 2 . Decomposition: If you're given two elements...
- Tue Oct 11, 2016 10:17 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Questions on the two exceptions, Chromium and Copper. [ENDORSED]
- Replies: 3
- Views: 860
Re: Questions on the two exceptions, Chromium and Copper. [ENDORSED]
If you look closely, when it jumps from 3d 3 (V) to 3d 5 (Cr), the 4s 2 changes to 4s 1 . So it seems that as you go from Vanadium to Chromium, one of the electrons from 4s moves to 3d (since 3d 5 and 3d 10 subshells have lower energy) and another electron is added, turning it from 3d 3 4s 2 to 3d 5...
- Sun Oct 09, 2016 12:42 am
- Forum: Properties of Light
- Topic: Properties of Light [ENDORSED]
- Replies: 1
- Views: 465
Re: Properties of Light [ENDORSED]
Many of light's behaviours can be explained by the wave model (such as diffraction with the diffraction patterns), but it is the photoelectric effect in which we can only explain light's behaviour with the particle model (photons). I'm not sure if it would be exactly correct to think of it as light ...
- Sun Oct 09, 2016 12:01 am
- Forum: Balancing Chemical Reactions
- Topic: Whole Numbers [ENDORSED]
- Replies: 1
- Views: 557
Re: Whole Numbers [ENDORSED]
I've seen equations being written with numbers like 3.5 or 0.5, but it's probably best to have them all in whole numbers. So yeah, you'd multiply by 2 to get whole numbers.
- Fri Oct 07, 2016 11:27 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Post Module Heisenberg #11 [ENDORSED]
- Replies: 4
- Views: 1111
Re: Post Module Heisenberg #11 [ENDORSED]
For per mole, all you have to do is multiply the value of Joules per electron by Avogadro's number, 6.022*10 23 atoms(in this case, electrons)/mol. Since this atom is Hydrogen, 1 atom has 1 electron, so getting to electrons/mol using dimensional analysis: 6.022*10 23 atoms/mol * 1 electron/1 atom (o...
- Fri Oct 07, 2016 11:17 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg's Indeterminacy Equation Clarification [ENDORSED]
- Replies: 3
- Views: 997
Re: Heisenberg's Indeterminacy Equation Clarification [ENDORSED]
Schrodinger's equation basically combined the concepts of De Broglie's equation and the Heisenberg's indeterminancy equation into one formula. Schrodinger wanted an equation that would allow people to find the energy of an electron by describing the electron as a wave function without having to use ...
- Fri Oct 07, 2016 6:49 pm
- Forum: Photoelectric Effect
- Topic: Stern and Gerlach Experiment [ENDORSED]
- Replies: 2
- Views: 948
Re: Stern and Gerlach Experiment [ENDORSED]
As seen in the course reader page 62, the set up was to have an atom beam of silver atoms going through a magnetic field, which would hit a collection plate on the other side. What happened was that the atoms (which were in their ground state with their 1 unpaired electron) were accumulating on two ...
- Fri Oct 07, 2016 4:58 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Pauli Exclusion Principle [ENDORSED]
- Replies: 1
- Views: 638
Re: Pauli Exclusion Principle [ENDORSED]
Yes, 2 electrons can both be in 1 orbital (m l ) only as long as they are paired (have different spins, AKA different m s values). They cannot have the same spin (both +1/2 or both -1/2), if they're to inhabit the same orbital. Pauli's exclusion principle also states that each orbital can only have ...
- Fri Oct 07, 2016 4:38 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg's Indeterminacy Equation Clarification [ENDORSED]
- Replies: 3
- Views: 997
Re: Heisenberg's Indeterminacy Equation Clarification [ENDORSED]
\Delta p = uncertainty in momentum, \Delta x = uncertainty in position The worked examples actually don't ask for position; they actually give us the uncertainty in position \Delta x (1st example gives us 1.7*10 -15 m, and the 2nd example gives us 2.5*10 -10 m). The examples don't actually directly...
- Fri Oct 07, 2016 3:01 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Hund's Rule confusion [ENDORSED]
- Replies: 1
- Views: 578
Re: Hund's Rule confusion [ENDORSED]
So it means that the orbitals (m l ) of the subshells (l) need to have the same spin (m s ). For example, in the 2p subshell, there are the orbitals 2p x , 2p y , and 2p z . According to Hund's Rule, you need to have the same m s number for each of these orbitals, which would be either +1/2 or -1/2....
- Fri Oct 07, 2016 2:55 pm
- Forum: Properties of Electrons
- Topic: Electron Configurations of Atoms [ENDORSED]
- Replies: 1
- Views: 439
Re: Electron Configurations of Atoms [ENDORSED]
Electrons in the same orbital (m l ) cannot have the same spin (m s ). So m s for the two electrons can't be both +1/2 or -1/2. So for example, the 2 electrons that occupy the orbital (m l ) 2p x cannot both have m s value +1/2. There has to be one of each +1/2 and -1/2. This is what it means to be ...
- Thu Oct 06, 2016 8:34 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty in Velocity? [ENDORSED]
- Replies: 1
- Views: 460
Re: Uncertainty in Velocity? [ENDORSED]
I believe when you're using the value in the indeterminancy equation, you use double 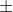 [whatever the number is], if it's given in this format. Like you said, yea you'd double the amount and use that value, and it would be the spread.
[whatever the number is], if it's given in this format. Like you said, yea you'd double the amount and use that value, and it would be the spread.
So,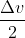 =
= 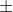 [whatever number]
[whatever number]
So,
- Thu Oct 06, 2016 8:00 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: wave functions and the heisenburg uncertainty [ENDORSED]
- Replies: 1
- Views: 503
Re: wave functions and the heisenburg uncertainty [ENDORSED]
The Heisenberg indeterminancy equation shows us that there is no way for us to know both the exact position and momentum of an electron at a certain point in time, and that we can only know the "range" of the possible positions and momentum the electron could be at. \psi 2 shows the probab...
- Thu Oct 06, 2016 6:15 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminacy Equation
- Replies: 1
- Views: 461
Re: Indeterminacy Equation
It wouldn't be the electron with the diameter of 10 -15 but rather the atom's diameter (AKA in this case the "range" of the possible positions the electrons could be in). If the atom's diameter is less than 10 -15 , then yes it would be unrealistic. I'm not entirely too sure on the "u...
- Thu Oct 06, 2016 5:58 pm
- Forum: *Shrodinger Equation
- Topic: Wave function [ENDORSED]
- Replies: 1
- Views: 627
Re: Wave function [ENDORSED]
A wave function ( \psi ), the way I understand it, is a function that models a wave. You can model waves with the sin and cos functions (sin and cos really only differ by their phases, and derivative of sin(x) -> cos(x), and derivative of cos(x) -> -sin(x)). Schrodinger, in a way, combined the De Br...
- Thu Oct 06, 2016 11:40 am
- Forum: *Shrodinger Equation
- Topic: Relation to sin and cos [ENDORSED]
- Replies: 1
- Views: 712
Re: Relation to sin and cos [ENDORSED]
Schrodinger basically used a wave function to describe an electron so he could come up with a general equation for finding the energy of an electron instead of having just an empirical equation like the Rydberg equation. Wave functions are basically talking about the sin/cos functions. And, the deri...
- Fri Sep 30, 2016 4:27 pm
- Forum: Student Social/Study Group
- Topic: Sunset Village Study Group
- Replies: 17
- Views: 3588
Re: Sunset Village Study Group
Hey! I'm also in B1, so I'd be interested as well!
- Thu Sep 29, 2016 2:39 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: The Number of Moles in 2Na+2H2O--> 2NaOH+H2?
- Replies: 3
- Views: 30537
Re: The Number of Moles in 2Na+2H2O--> 2NaOH+H2?
Hey, Coefficients in chemical equations do not strictly refer to moles. More generally, it tells us the ratio of reactants needed to produce products. It could both mean the number of individual atoms/molecules of product and reactants, and the number of moles of product and reactants. Most of the t...

