Search found 50 matches
- Sun Mar 05, 2017 8:52 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Quiz 3 Winter 2017
- Replies: 183
- Views: 29669
Re: Quiz 3 Winter 2017
Can someone help me with #3 I don't understand how both of them are nucleophiles? The line diagram has multiple double bonds which are made up of a pi bond. These pi bonds serve as areas of high electronegativity due to their being so many localized electrons at each pi bond. This make it attracted...
- Thu Mar 02, 2017 3:18 pm
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Catalysts/Enzymes regarding Entropy
- Replies: 1
- Views: 1344
Catalysts/Enzymes regarding Entropy
Entropy is a measure of disorder, so if you make delta S less positive, you decrease entropy (more order), and if you make delta S less negative, you increase entropy (more disorder), correct? In lecture, we were told that catalysts and enzymes speed a reaction by putting reactants in the correct or...
- Sun Feb 26, 2017 8:18 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Question 15.79
- Replies: 1
- Views: 462
Question 15.79
"In the reaction of Her with the reactive intermediate CH 3 CH=CHCH 2 + , at low temperatures the predominant product is CH3CHBrCH=CH2, but at high temperatures, the predominant product is CH 3 CH=CHCH 2 Br. (a) Which product is formed by the pathway with the larger activation energy? (b) Does ...
- Sat Feb 18, 2017 1:10 pm
- Forum: General Rate Laws
- Topic: Unique Average Rate --> Rate Law [ENDORSED]
- Replies: 2
- Views: 567
Unique Average Rate --> Rate Law [ENDORSED]
Page 60 of the course reader says that the reaction rate is -(1/a)*(dR/dt) = k[R]n. Is there any specific derivation to come to this conclusion, or is this just the definition of a reaction rate?
- Fri Feb 10, 2017 6:16 am
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Electrolysis: Water 1/2 Rxn
- Replies: 1
- Views: 526
Electrolysis: Water 1/2 Rxn
The textbook gives us: 2 H2(g) + O2(g) --> 2 H2O(l) Ecell = +1.23V at pH = 7 2 H2O(l) --> 2 H2(g) + O2(g) Ecell = -1.23V at pH = 7 O2(g) + 4 H+ (aq) + 4e- --> 2 H2O(l) E = +0.82V at pH = 7 How do we know when to use these equations as an anode or cathode reaction, and how do we determine which one t...
- Sun Feb 05, 2017 4:37 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Problem 14.25
- Replies: 3
- Views: 624
Problem 14.25
"Arrange the following metals in order of increasing strength as reducing agents for species in aqueous solution: (a) Cu, Zn, Cr, Fe (b) . . . " When looking up the standard reduction potentials in Appendix 2B, there are multiple values for some of the metals (i.e. Cu has E* values for Cu ...
- Thu Feb 02, 2017 2:01 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Gibbs free energy for cell rxns: UNITS
- Replies: 2
- Views: 541
Gibbs free energy for cell rxns: UNITS
When calculating delta G* using -nFE*, units cancel nicely to yield a result in units J. However, in previous exercises, delta G* has had units kJ.mol-1. I know you can convert J to kJ, but what are the correct units for delta G*?
- Sun Jan 29, 2017 2:20 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy/Equil Constant Relationship
- Replies: 1
- Views: 443
Gibbs Free Energy/Equil Constant Relationship
On page 41 of the course reader, it says delta G* is positive when K<1 and that delta G* is negative when K>1. Does that mean the forward reaction is not spontaneous when K<1 and is when K>1? I understand the arithmetic but I don't understand because if K<1, the reaction will want to go forward (spo...
- Sat Jan 28, 2017 8:13 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Problem 9.63
- Replies: 1
- Views: 419
Problem 9.63
Does a negative delta G* mean stability in addition to spontaneity? Why?
- Thu Jan 26, 2017 4:54 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Problem 9.47
- Replies: 1
- Views: 432
Problem 9.47
a) For Part (a), why was -w rev substituted in for q rev in the SSM? Also, will delta S tot always = 0 for reversible reactions? b) Part (b) asks to calculate delta S tot , delta S sys , and delta S surr for an isothermal, irreversible free expansion of an ideal gas beginning at 323K, 1.67L, and 4.9...
- Fri Jan 20, 2017 6:19 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Problem 8.51
- Replies: 1
- Views: 436
Problem 8.51
"The enthalpy of formation of TNT is -67 kJ.mol -1 , and the density of TNT is 1.65 g.cm -3 ...Explore its potential as a rocket fuel by calculating its enthalpy density (enthalpy released per liter) for the reaction 4C 7 H 5 N 3 O 6 (s) + 21 O 2 (g) --> 28 CO 2 (g) + 10 H 2 O(g) + 6 N 2 (g) I ...
- Fri Jan 13, 2017 7:02 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Problem 8.63c typo?
- Replies: 2
- Views: 486
Problem 8.63c typo?
Appendix 2A tells that delta hf* for K2S(aq) is -471.5. The answer in the textbook and SSM uses -417.5. Which is the correct delta hf* value?
- Fri Jan 13, 2017 6:29 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpy of Form; Balanced Eqns
- Replies: 2
- Views: 471
Re: Standard Enthalpy of Form; Balanced Eqns
Thank you! :)
- Thu Jan 12, 2017 4:31 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpy of Form; Balanced Eqns
- Replies: 2
- Views: 471
Standard Enthalpy of Form; Balanced Eqns
Why are fractional coefficients required when balancing the equation? Don't whole numbers make everything cleaner?
- Fri Dec 02, 2016 12:05 pm
- Forum: *Titrations & Titration Calculations
- Topic: 2012 Final Q7b
- Replies: 1
- Views: 420
2012 Final Q7b
"Calculate the pH at the stoichiometric point for the titration of 15.00mL of 0.100M HCOOH (K a = 1.8 x 10 -4 ) with 0.150M NaOH." Can someone please explain how to start this? I'm looking at the answer key and lecture notes, but I am completely lost on the logic of how to start and procee...
- Wed Nov 30, 2016 11:17 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 12.57, pH/pOH v. pKa/pKb
- Replies: 1
- Views: 617
12.57, pH/pOH v. pKa/pKb
Are pH=pKa and pOH=pKb? I am unsuccessfully trying to figure out why the answers the 12.57 are what they are. Part (a) gives tells that the pH of .10M HClO2 was measured to be 1.2 and asks us to to determine Ka and pKa. Isn't 1.2 the same as pKa?
- Tue Nov 29, 2016 8:39 am
- Forum: Bronsted Acids & Bases
- Topic: Textbook Q12.9c
- Replies: 1
- Views: 524
Textbook Q12.9c
Question 12.9c asks if the reaction CH3COOH(aq) + NH3(aq) ---> CH3CONH2 (aq) + H2O (l) can be classified as reaction between Bronsted acids and bases and to identify the acid and base if it as an acid-base reaction. Can someone please explain how no proton is transferred?
- Tue Nov 29, 2016 6:23 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Q11.93b
- Replies: 2
- Views: 608
Re: Textbook Q11.93b
Alright, thanks!
- Mon Nov 28, 2016 9:04 pm
- Forum: Bronsted Acids & Bases
- Topic: Common Acids/Bases
- Replies: 3
- Views: 738
Common Acids/Bases
Should we memorize the strong/weak acids and bases listed on pages 163-64 in the course reader?
- Mon Nov 28, 2016 7:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When to use atm v. bar units [ENDORSED]
- Replies: 4
- Views: 2024
Re: When to use atm v. bar units [ENDORSED]
Ok, thanks!
- Mon Nov 28, 2016 7:35 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Q11.93b
- Replies: 2
- Views: 608
Textbook Q11.93b
"0.020 mol NO 2 was introduced into a 1.00-L flask and the reaction 2 NO 2 (g) <--> N 2 O 4 (g) was allowed to come to equilibrium at 298 K. (a) Using information in Table 11.2, calculate the equilibrium concentrations of the two gases. (b) The volume of the flask is reduced to half its origina...
- Mon Nov 28, 2016 7:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When to use atm v. bar units [ENDORSED]
- Replies: 4
- Views: 2024
When to use atm v. bar units [ENDORSED]
Should we always use atm (unless specified in the question to use bar)? For instance, Q8 on p. 43 of the quiz workbook asks us to calculate the equilibrium partial pressures but does not specify which units to use. Q10 on the following page requires you to convert from molar concentration to partial...
- Mon Nov 28, 2016 10:53 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Quiz Workbook P 49 Q9
- Replies: 2
- Views: 727
Re: Quiz Workbook P 49 Q9
Great, thank you!
- Mon Nov 28, 2016 10:34 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Quiz Workbook P 44 Q9
- Replies: 2
- Views: 3688
Re: Quiz Workbook P 44 Q9
Got it, thank you!
- Sun Nov 27, 2016 8:09 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Quiz Workbook P 44 Q9
- Replies: 2
- Views: 3688
Quiz Workbook P 44 Q9
Is this the type of question where it gives you too much information (are K and the molar concentrations necessary)? Do you even need to use an ICE box?
- Sun Nov 27, 2016 8:05 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Quiz Workbook P 49 Q9
- Replies: 2
- Views: 727
Quiz Workbook P 49 Q9
My answer and the answer key do not match at all, but I don't know where I went wrong! Can someone please explain how to find K if 8.15% of the reactant remains at equilibrium? Thank you!
- Sat Nov 26, 2016 3:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: H2O [ENDORSED]
- Replies: 2
- Views: 705
Re: H2O [ENDORSED]
Liquids and solids are pure substances, and pure substances are not included in the calculation.
Amine
If NH3 is amine, how come there are only 2 Hs for each amine in en and dien?
- Thu Nov 10, 2016 2:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ch 11 #7c
- Replies: 2
- Views: 654
Ch 11 #7c
The SSM uses the initial pressure value for both the numerator and denominator; how? Should we assume that the pressure is constant?
- Mon Nov 07, 2016 10:24 am
- Forum: Naming
- Topic: order of ligand names [ENDORSED]
- Replies: 7
- Views: 1665
Re: order of ligand names [ENDORSED]
I notice that when water is a ligand (looking at #31), the SSM writes its formula as H2O for one exercise and OH2 for another one. I know the order of the atoms matter, so which one is correct? Why?
- Mon Nov 07, 2016 6:26 am
- Forum: Naming
- Topic: Number 29 from the Hw [ENDORSED]
- Replies: 4
- Views: 810
Re: Number 29 from the Hw [ENDORSED]
Finding the oxidation number of metals is generally pretty straightforward as you would just subtract the overall charge of the compound by any negatively charged ligands located between the brackets (for the iron compound, there are 6 CN - molecules so subtract that from -4 gives a 2+ charge for t...
- Thu Nov 03, 2016 5:52 pm
- Forum: Electronegativity
- Topic: Problem 4.57
- Replies: 3
- Views: 868
Re: Problem 4.57
So, generally speaking, ionization energy and electronegativity are directly related (IE and EN increase across a period), but oxygen is an exception since it has one e- over a half-filled orbital?
- Wed Nov 02, 2016 1:46 pm
- Forum: Hybridization
- Topic: 2014 Midterm Q8
- Replies: 5
- Views: 900
Re: 2014 Midterm Q8
Oh wait, I understand now, thank you!
- Wed Nov 02, 2016 12:41 pm
- Forum: Hybridization
- Topic: 2014 Midterm Q8
- Replies: 5
- Views: 900
Re: 2014 Midterm Q8
So are pi bonds always not hybridized?
- Tue Nov 01, 2016 10:25 am
- Forum: Hybridization
- Topic: 2014 Midterm Q8
- Replies: 5
- Views: 900
2014 Midterm Q8
For double bonding, why are the overlapping orbitals that form a pi bond not hybridized?
- Tue Nov 01, 2016 10:11 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: 2012 Midterm Q3c
- Replies: 2
- Views: 745
2012 Midterm Q3c
Are we expected to know what wavelength values each atomic spectra region includes?
- Tue Nov 01, 2016 8:59 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Molecular Orbital theory
- Replies: 5
- Views: 1012
Re: Molecular Orbital theory
The midterm covers only up to the end of hybridization, so there is no MO Theory.
- Tue Nov 01, 2016 8:13 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Question 4.61
- Replies: 1
- Views: 268
Question 4.61
When you have a heteronuclear diatomic molecule with one Z > 8 and the other Z < 8, how do you know when the pi bonding orbital comes before the sigma bonding orbital, and vice versa? i.e. CO
- Tue Nov 01, 2016 8:03 am
- Forum: Electronegativity
- Topic: Problem 4.57
- Replies: 3
- Views: 868
Problem 4.57
How is oxygen more electronegative than nitrogen if it has a lower ionization energy?
- Mon Oct 31, 2016 11:00 pm
- Forum: Lewis Structures
- Topic: 2012 Midterm Q5b, part (e)
- Replies: 2
- Views: 414
Re: 2012 Midterm Q5b, part (e)
That's right, thank you!
- Mon Oct 31, 2016 6:11 pm
- Forum: Lewis Structures
- Topic: 2012 Midterm Q5b, part (e)
- Replies: 2
- Views: 414
2012 Midterm Q5b, part (e)
Why is N the central atom for N2O? Doesn't O have the lower ionization energy?
- Mon Oct 31, 2016 5:57 pm
- Forum: Lewis Structures
- Topic: Lewis acids and bases [ENDORSED]
- Replies: 2
- Views: 661
Re: Lewis acids and bases [ENDORSED]
I understand now, thank you!
- Mon Oct 31, 2016 11:46 am
- Forum: Hybridization
- Topic: Hybrid Orbital combinations
- Replies: 2
- Views: 346
Re: Hybrid Orbital combinations
Great, thank you!
- Mon Oct 31, 2016 11:45 am
- Forum: Lewis Structures
- Topic: Lewis acids and bases [ENDORSED]
- Replies: 2
- Views: 661
Lewis acids and bases [ENDORSED]
How can you determine whether or not an element is a Lewis acid or base? For instance, for AlCl3, is Al donating e-s to Cl so that Cl has a full octet, therefore making it a Lewis base?
- Fri Oct 28, 2016 10:40 am
- Forum: Hybridization
- Topic: Hybrid Orbital combinations
- Replies: 2
- Views: 346
Hybrid Orbital combinations
I do not understand how hybrid combinations are derived. For instance, the 4 hybrid orbitals of sp3 are:
h1 = s + px + py + pz
h2 = s - px - py + pz
h3 = s - px + py - pz
h4 = s + px - py - pz
Are these fundamental? Will we be expected to know these for the midterm?
h1 = s + px + py + pz
h2 = s - px - py + pz
h3 = s - px + py - pz
h4 = s + px - py - pz
Are these fundamental? Will we be expected to know these for the midterm?
- Thu Oct 20, 2016 10:22 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Problem 3.65
- Replies: 1
- Views: 502
Problem 3.65
How is possible for elements in Group 18 to form bonds (don't they already have a filled octet)?
- Tue Oct 18, 2016 5:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: Question 3.39b [ENDORSED]
- Replies: 1
- Views: 405
Question 3.39b [ENDORSED]
Can someone please explain why P is the central atom of the Lewis structure? It makes sense on paper because it looks nice, but doesn't K have the lower ionization energy?
- Wed Oct 12, 2016 9:55 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Quantum Numbers n, l, and m
- Replies: 2
- Views: 553
Quantum Numbers n, l, and m
I am very confused on the concept on n, l, and m l ... 1) Can the relationship between n, l, and m be described as shell, sub-shell, and orbital within a sub-shell, respectively? 2) Does the l-value tell you the highest possible sub-shell of that particular energy level? (i.e. n=3; l=0, 1, & 2 -...
- Wed Oct 05, 2016 6:25 pm
- Forum: Properties of Electrons
- Topic: Difficulty with Problem 1.55 [ENDORSED]
- Replies: 1
- Views: 443
Re: Difficulty with Problem 1.55 [ENDORSED]
I too, at first, converted the cm into m and got a different answer from the SSM. I re-read the problem, and it is says that it's "common to express energy in terms of v/c with units cm -1 " (for this particular kind of spectroscopy). So literally all you have to do is set up v/c = some va...
- Wed Sep 28, 2016 9:28 am
- Forum: SI Units, Unit Conversions
- Topic: H-Atom Electron in Energy Level n
- Replies: 1
- Views: 270
H-Atom Electron in Energy Level n
In the Atomic Spectra and The Bohr Frequency Condition module, we derived an equation that resulted in 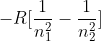 . In the book and SSM, R is positive, not negative. Which equation is correct?
. In the book and SSM, R is positive, not negative. Which equation is correct?

