Search found 36 matches
- Fri Mar 17, 2017 9:28 pm
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Substituents, naming Z or E
- Replies: 1
- Views: 1337
Re: Substituents, naming Z or E
yup, you're right
- Tue Mar 14, 2017 12:13 am
- Forum: Calculating Work of Expansion
- Topic: Reversible vs irreversible equations
- Replies: 3
- Views: 848
Re: Reversible vs irreversible equations
I'm confused about your equation for adiabatic processes. I thought 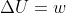 for adiabatic processes.
for adiabatic processes.
Thanks for the help Dr. Lavelle!
Thanks for the help Dr. Lavelle!
- Fri Mar 10, 2017 3:58 pm
- Forum: *Cycloalkenes
- Topic: Numbering carbon atoms
- Replies: 1
- Views: 1329
Re: Numbering carbon atoms
I'm not sure if I am understanding your question, but we do look for the longest chain even if there is a cycloalkane. For example, if there is a decane with a cyclopropane attached to the 2nd carbon, it would be called 2-cyclopropyl-decane. Let me know if you have more questions or if I misundersto...
- Sun Mar 05, 2017 9:16 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: Functional Groups
- Replies: 1
- Views: 516
Re: Functional Groups
This is because by definition, aldehydes only have 1 R group to connect to, so it must be at the end. On the other hand, ketones, by definition, must connect to 2 R groups, so it cannot be at the end of a carbon chain.
- Sun Mar 05, 2017 7:48 pm
- Forum: *Cycloalkanes
- Topic: Prefix in Naming
- Replies: 1
- Views: 563
Re: Prefix in Naming
Tert describes the structure of the substituent. Here it shows that the butyl group has a tertiary carbon.
- Sat Feb 25, 2017 3:14 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590976
- Sun Feb 19, 2017 8:45 pm
- Forum: General Rate Laws
- Topic: Derivation for the Differential Rate Law
- Replies: 3
- Views: 720
Re: Derivation for the Differential Rate Law
Actually I think I understand it now.
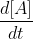 is just the general term for the instantaneous rate and
is just the general term for the instantaneous rate and 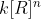 is just the experimentally determined rate at which the reaction occurs.
is just the experimentally determined rate at which the reaction occurs.
- Sun Feb 19, 2017 8:03 pm
- Forum: General Rate Laws
- Topic: Derivation for the Differential Rate Law
- Replies: 3
- Views: 720
Re: Derivation for the Differential Rate Law
This is page 60.
Thanks for the help!
- Sun Feb 19, 2017 1:43 am
- Forum: General Rate Laws
- Topic: Derivation for the Differential Rate Law
- Replies: 3
- Views: 720
Derivation for the Differential Rate Law
The course reader was very clear on how the integrated rate law is derived. However, I am confused about how the differential rate law is derived and neither the course reader nor the textbook explicitly show this. The course reader states RATE = -\frac{1}{a}\frac{d[R]}{dt} = k[R]^{n} , but I am uns...
- Sat Feb 18, 2017 1:39 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590976
Re: Post All Chemistry Jokes Here
Did you hear about the man who got cooled to absolute zero?
He's 0K now.
He's 0K now.
- Wed Feb 15, 2017 4:18 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Winter Midterm 2014 QB
- Replies: 3
- Views: 785
Re: Winter Midterm 2014 QB
I assumed it was hexane because it has more isomers, but I am also unsure.
- Sat Feb 11, 2017 1:26 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590976
- Fri Feb 03, 2017 9:09 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: The Salt Bridge
- Replies: 1
- Views: 493
Re: The Salt Bridge
If Cl- stays in the cathode, the cathode would be negatively charged, so electrons would be repelled from the cathode. The salt bridge allows these ions to move to the anode and make the 2 solutions neutral in charge. About electronegativty: Chlorine itself is electronegative so it takes an electron...
- Fri Jan 27, 2017 1:25 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy when P isn't constant
- Replies: 1
- Views: 477
Re: Entropy when P isn't constant
Hope this helps!
- Sun Jan 22, 2017 8:12 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Different ways to calculate reaction enthalpies
- Replies: 1
- Views: 504
Re: Different ways to calculate reaction enthalpies
My TA told me that for the other methods, we should assume that the information given is at the temperature of the reaction enthalpy. For example, if you need to find the reaction enthalpy at 30ºC using Hess's law, the other reactions must also be at 30ºC. I do not believe temperature change can be ...
- Fri Jan 20, 2017 9:15 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: General questions [ENDORSED]
- Replies: 4
- Views: 1149
Re: General questions [ENDORSED]
I know number 1.
1. NA (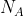 ) is Avogradro's number, but it does represent the number of particles.
) is Avogradro's number, but it does represent the number of particles.
Following for the others.
1. NA (
Following for the others.
- Mon Jan 16, 2017 11:52 pm
- Forum: Phase Changes & Related Calculations
- Topic: Boiling Points at Altitude [ENDORSED]
- Replies: 2
- Views: 686
Re: Boiling Points at Altitude [ENDORSED]
Water boils at a lower temperature at lower pressures. This is because the atmospheric pressure is lower, so less vapor pressure (created by increasing temperature) is needed for the gas to escape.
- Thu Dec 01, 2016 3:01 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Question 12.23 (Kw not at STP) [ENDORSED]
- Replies: 1
- Views: 696
Question 12.23 (Kw not at STP) [ENDORSED]
Question 12.23 asks to find the pH of neutral water at 37C and gives the Kw=2.1X10^-14. The answer is pH=6.80. What I'm confused about is the hydronium ion concentration is equal to the hydroxide ion concentration, so if we take the -log of [OH-], pOH = 6.8. Does pH+pOH=14 not apply to systems not a...
- Thu Dec 01, 2016 11:12 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier
- Replies: 3
- Views: 868
Re: Le Chatelier
That would be the equilibrium constant. For example, if 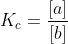 and you add more B, then A would increase and B would decrease until you reach the equilibrium constant.
and you add more B, then A would increase and B would decrease until you reach the equilibrium constant.
- Fri Nov 25, 2016 5:18 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Indicators of strong or weak acid/base
- Replies: 3
- Views: 852
Re: Indicators of strong or weak acid/base
A simple indicator is if the question gives you the Ka or Kb, you should assume that it is a weak acid/base. This is because a strong acid/base has a very large Ka/Kb, so we just say that the reaction goes to completion and the acid is fully dissociated (HCl fully becomes H+ and Cl-).
- Thu Nov 17, 2016 1:20 am
- Forum: Naming
- Topic: Quizlet for the names of Common Ligands
- Replies: 10
- Views: 2237
Re: Quizlet for the names of Common Ligands
I'm sure we need to know them for Quiz 3. I'm pretty sure we need to know them for the final too.
- Thu Nov 17, 2016 1:01 am
- Forum: Naming
- Topic: Homework 17.31 (d): Naming Alphabetically
- Replies: 2
- Views: 684
Re: Homework 17.31 (d): Naming Alphabetically
You should alphabetize according to the actual ligand name, not the prefixes. Aqua is before oxalato in alphabetic order, so diaqua comes first.
- Fri Nov 11, 2016 4:28 pm
- Forum: Naming
- Topic: Asterisks on the Table of Ligands [ENDORSED]
- Replies: 4
- Views: 1094
Re: Asterisks on the Table of Ligands [ENDORSED]
I don't think the asterisks mean that it is a polydentate because neither nitrito nor sulfato are polydentates, but they have asterisks.
I don't know what the asterisks mean either, so I'm following this thread.
I don't know what the asterisks mean either, so I'm following this thread.
- Thu Nov 10, 2016 11:22 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chapter 17 Question #33
- Replies: 1
- Views: 426
Re: Chapter 17 Question #33
What you can do to figure this out is draw the lewis structures for each of the ligands and find how many areas of high electron density there are to determine the number of binding sites. If the number of binding sites is greater than 1, it is a polydentate. Additionally, you can use the informatio...
- Thu Nov 10, 2016 11:18 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Molecular orbitals
- Replies: 1
- Views: 432
Re: Molecular orbitals
MO orbitals describe the electrons in an entire molecule while hybrid orbitals describe the electrons around a single atom. They can and often do appear together, but the MO diagram only shows the energies of the electrons while we can determine the shape of the areas of electron density from what w...
- Sun Nov 06, 2016 11:54 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590976
Re: Post All Chemistry Jokes Here
If H20 is water, what is H204?
Drinking, bathing, washing, swimming, etc.
Drinking, bathing, washing, swimming, etc.
- Fri Oct 28, 2016 3:00 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590976
Re: Post All Chemistry Jokes Here
I hate it when I make a chemistry joke, but there's no reaction.
- Sun Oct 23, 2016 5:01 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590976
Re: Post All Chemistry Jokes Here
Chemistry version of Pen Pineapple Apple Pen. So dumb, yet so funny!
https://www.youtube.com/watch?v=nqL75fASb6U
https://www.youtube.com/watch?v=nqL75fASb6U
- Sat Oct 15, 2016 2:49 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 2.21
- Replies: 5
- Views: 551
Re: 2.21
l=0 is the s orbital
l=1 is the p orbital
l=2 is the d orbital
l=3 is the f orbital
If you have any more questions let me know!
l=1 is the p orbital
l=2 is the d orbital
l=3 is the f orbital
If you have any more questions let me know!
- Sun Oct 09, 2016 11:34 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Homework 1.13 [ENDORSED]
- Replies: 3
- Views: 818
Re: Homework 1.13 [ENDORSED]
Sorry for the late reply, but R is Rhydberg's constant and 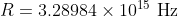
- Sun Oct 09, 2016 10:53 pm
- Forum: SI Units, Unit Conversions
- Topic: Sig Figs [ENDORSED]
- Replies: 16
- Views: 3269
Re: Sig Figs [ENDORSED]
3
This can be seen more easily if you convert this to scientific notation.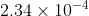
This can be seen more easily if you convert this to scientific notation.
- Sun Oct 09, 2016 10:52 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Quiz 1 Preparation: Fall 2014 Question #10 [ENDORSED]
- Replies: 6
- Views: 1344
Re: Quiz 1 Preparation: Fall 2014 Question #10 [ENDORSED]
I didn't get rid of the negative, the number that is being square rooted is positive. I first used the equation E = h\nu to find the energy. Then I used \Delta E = E_{final} - E_{initial} where E_{n}=\frac{-hr}{n^{2}} . You just equations that have variables you know and variables you need to find, ...
- Sun Oct 09, 2016 8:57 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Quiz 1 Preparation: Fall 2014 Question #10 [ENDORSED]
- Replies: 6
- Views: 1344
Re: Quiz 1 Preparation: Fall 2014 Question #10 [ENDORSED]
Here's my work, but I didn't include every step, so if you have any questions about it, please let me know.
Hope this helps! :)
-Alexander Chen
Hope this helps! :)
-Alexander Chen
- Thu Oct 06, 2016 12:02 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Homework 1.13 [ENDORSED]
- Replies: 3
- Views: 818
Re: Homework 1.13 [ENDORSED]
Hey Grace!
I worked out the problem for you in the picture below.
Let me know if you have any questions!
I worked out the problem for you in the picture below.
Let me know if you have any questions!
- Thu Oct 06, 2016 11:47 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Video Module Question
- Replies: 2
- Views: 619
Re: Atomic Spectra Video Module Question
Hi Raj!
I just went through this question and got that the principle quantum level is 6.
Attached is a picture of my work.
Let me know if you have any more questions!
I just went through this question and got that the principle quantum level is 6.
Attached is a picture of my work.
Let me know if you have any more questions!
- Wed Sep 28, 2016 7:31 pm
- Forum: General Science Questions
- Topic: Memorizing Constants
- Replies: 2
- Views: 836
Re: Memorizing Constants
I believe Dr. Lavelle said we would have a formula sheet during exams that will give us the formulas and constants that we would need. The only thing you would have to know is when to use what, because each question will not tell you the constant or formula you need to solve the question.

