Search found 16 matches
- Wed Mar 15, 2017 8:17 pm
- Forum: *Cyclopropanes and Cyclobutanes
- Topic: How to chair flip?
- Replies: 1
- Views: 1298
Re: How to chair flip?
When doing a flip, all the axial ups become equatorial ups and the axial downs become equatorial downs.
- Thu Mar 09, 2017 5:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Excellence in Chemistry Award 2014-15
- Replies: 5
- Views: 1435
- Thu Mar 09, 2017 10:53 am
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Standard Gibbs Free Energy of Activation
- Replies: 4
- Views: 1641
Standard Gibbs Free Energy of Activation
Why is standard Gibbs free energy of activation always a positive value, in terms of conceptual understanding?
- Sun Mar 05, 2017 8:58 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Chapter 15: Question #99
- Replies: 1
- Views: 419
Re: Chapter 15: Question #99
For a majority of these, you can look at the corresponding formula on the formula sheet, and if the formula is in a y=mx+b form, you know it will be linear. For instance, B is linear because [R]=-kt + [R](initial) because this fits the y=mx+b form for [R] against time regarding a zero-order reaction...
- Sun Feb 26, 2017 7:40 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: "No charge separation" meaning? [ENDORSED]
- Replies: 3
- Views: 11864
Re: "No charge separation" meaning? [ENDORSED]
Recall from Chem 14A the concepts of Resonance and Formal Charge. The better resonance structure would have the lower formal charge. Formal charge is calculated by (# of Valence Electrons) - (# of non-bonding electrons + ((1/2)(bonding electrons)). So the no charge separation refers to the resonance...
- Thu Feb 23, 2017 11:08 am
- Forum: First Order Reactions
- Topic: Practice Quiz 2 #9
- Replies: 1
- Views: 513
Re: Practice Quiz 2 #9
Section 15.16 in the book covers Enzymes
- Thu Feb 23, 2017 11:06 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Homework Question 15.23
- Replies: 1
- Views: 470
Re: Homework Question 15.23
We use stoichiometry to find our concentration of A. The reaction is 2A \rightarrow B + C We can find the final concentration of A since we are given the final concentration of B. So, .034mol B/L * (2 mol A/1 mol B)=.068 mol of A. Since we know our initial amount of A was 0.153 mol, we subtract 0.15...
- Sat Feb 18, 2017 9:59 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate of Reaction vs. Reaction Rate Constant [ENDORSED]
- Replies: 2
- Views: 588
Re: Rate of Reaction vs. Reaction Rate Constant [ENDORSED]
The rate of reaction tells us how the rate is related to concentrations, since by definition it is a change in concentration of reactants over time, while the reaction rate constant depends on temperature and activation energy, which is why we can calculate the reaction rate constant using any conce...
- Sun Feb 12, 2017 12:14 am
- Forum: Balancing Redox Reactions
- Topic: Question 14.25
- Replies: 2
- Views: 500
Re: Question 14.25
For this question, look in appendix 2B and look for the most negative standard reduction potentials. Since we subtract cathode - anode, we want the most negative standard reduction potential in order to have a more positive E and therefore stronger reducing agent.
- Sun Feb 05, 2017 6:30 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3657181
Re: Post All Chemistry Jokes Here
Happy Week 5!
[img]/img]
[img]/img]
- Sun Feb 05, 2017 6:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: max cell potential
- Replies: 1
- Views: 667
Re: max cell potential
The equation 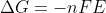 shows us that the free energy between the reactants and products (
shows us that the free energy between the reactants and products ( )
)
is directly related to the maximum cell potential (E). If you want to better understand how this equation is derived, page 49 in the course reader shows us in a lot of detail!
is directly related to the maximum cell potential (E). If you want to better understand how this equation is derived, page 49 in the course reader shows us in a lot of detail!
- Fri Jan 27, 2017 1:52 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy vs. Internal Energy
- Replies: 1
- Views: 1858
Gibbs Free Energy vs. Internal Energy
Can someone explain the difference between Gibbs free energy and internal energy to better conceptually understand the two?
- Sun Jan 22, 2017 2:40 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Textbook Problem 8.21
- Replies: 2
- Views: 580
Re: Textbook Problem 8.21
Keep in mind that q(system)+q(surroundings) = q(universe), which = 0 So, the heat lost by the metal = - heat gained by the water You can use q=mC(s)(T(final) - T(initial)) in place of the metal and the water, as seen with the set up of the equation above. Plug in the information given, then you will...
- Sun Jan 22, 2017 2:30 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HW 8.47
- Replies: 2
- Views: 618
Re: HW 8.47
Plug in this info and you will get,
- Fri Jan 13, 2017 10:10 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpies of Formation Basics
- Replies: 2
- Views: 565
Re: Standard Enthalpies of Formation Basics
A helpful way to remember the diatomic molecules is with the following saying: Have No Fear Of Ice Cold Beer
H=H2
N=N2
F=F2
O=O2
I=I2 (solid)
C=Cl2
B=Br2 (liquid)
H=H2
N=N2
F=F2
O=O2
I=I2 (solid)
C=Cl2
B=Br2 (liquid)
- Fri Jan 13, 2017 9:58 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calculating Heat Required
- Replies: 3
- Views: 811
Re: Calculating Heat Required
Note the Qp and just the Q in the second equation. The first equation takes into account constant pressure, while Q in the second equation is heat that is not at constant pressure.

