Search found 13 matches
- Fri Mar 17, 2017 12:52 am
- Forum: Balancing Redox Reactions
- Topic: Q4 2013 Practice Final
- Replies: 2
- Views: 611
Re: Q4 2013 Practice Final
Here, "n" represents the number of electrons in the balanced half reactions. In this question, our half reactions are: (Fe \rightarrow Fe^{2+} + 2e^{-}) \times 2 <-- we multiply this one by 2 to balance out the electrons and 4e^{-} + 2H_{2}O + O_{2} \rightarrow 4OH^{-} Hope that he...
- Mon Mar 13, 2017 12:20 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Unit of Equilibrium Constant K
- Replies: 1
- Views: 594
Unit of Equilibrium Constant K
Hello, I have a question regarding one of the questions in quiz 2. The question is: Consider the following reaction at equilibrium. A\rightleftharpoons B+C The equilibrium concentration were measured as follows: [A] = 0.0100M, [B] = 0.500M, [C] = 2.50M In a kinetic experiment, the rate constant of t...
- Thu Mar 09, 2017 10:11 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590570
Re: Post All Chemistry Jokes Here
ATTENTION! A new element has been discovered!
- Mon Mar 06, 2017 7:06 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590570
Re: Post All Chemistry Jokes Here
I hate dealing with triple bonds. They cause alkyne-ds of problems.
- Sun Mar 05, 2017 4:49 pm
- Forum: *Cycloalkenes
- Topic: Cyclopropane
- Replies: 1
- Views: 456
Re: Cyclopropane
It cannot be cyclopropane, because all cycloalkanes have the general molecular formula of 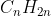
Meanwhile, CH3-CH2-CH3 has 3 carbons and 8 hydrogens, so it is a propane, which follows the general formula of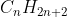 for alkanes.
for alkanes.
Meanwhile, CH3-CH2-CH3 has 3 carbons and 8 hydrogens, so it is a propane, which follows the general formula of
- Mon Feb 20, 2017 9:59 pm
- Forum: General Rate Laws
- Topic: Reaction Rate Units
- Replies: 1
- Views: 366
Re: Reaction Rate Units
No, the units for reaction rates do not change. They remain as M/sec or M/min (depending on what unit you use to measure time)
On the other hand, the units for rate constants (k) do change depending on the order of the reaction.
On the other hand, the units for rate constants (k) do change depending on the order of the reaction.
- Sun Feb 19, 2017 6:46 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation energy and its reliance on temperature
- Replies: 3
- Views: 742
Re: Activation energy and its reliance on temperature
Ea and temperature are related by the Arrhenius equation:
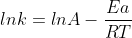
The higher the activation energy, the stronger is the temperature dependence of the rate constant k.
The higher the activation energy, the stronger is the temperature dependence of the rate constant k.
- Sat Feb 11, 2017 11:49 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation at 25 degrees? [ENDORSED]
- Replies: 1
- Views: 3341
Re: Nernst Equation at 25 degrees? [ENDORSED]
The "2.303" comes from writing the Nernst equation in log base 10 rather than in ln:
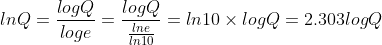
It's sometimes more convenient to use log base 10, like when you're dealing with pH.
It's sometimes more convenient to use log base 10, like when you're dealing with pH.
- Sat Feb 11, 2017 8:22 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Midterm 2014 Winter Q5A
- Replies: 2
- Views: 693
Re: Midterm 2014 Winter Q5A
Yeah, I'm also getting 1.21 x 10^-22.
Perhaps there's an error in the course reader?
Perhaps there's an error in the course reader?
- Sat Feb 04, 2017 3:04 pm
- Forum: Balancing Redox Reactions
- Topic: Easy way to remember when electrons are lost/gained
- Replies: 4
- Views: 2413
Re: Easy way to remember when electrons are lost/gained
My high school chemistry teacher taught me this trick to remember that reduction takes place at the cathode and oxidation takes place at the anode.
reduction, cathode = RED CAT
anode, oxidation = AN OX
reduction, cathode = RED CAT
anode, oxidation = AN OX
- Sun Jan 29, 2017 1:20 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: bomb calorimeter mechanism
- Replies: 1
- Views: 926
Re: bomb calorimeter mechanism
A bomb calorimeter measures the heat released in a reaction. A sample is placed in the sealed metal container (the "bomb"), which is immersed in water. The bomb is ignited electrically by a fuse wire, allowing the sample inside to combust. Once combustion has begun, energy released as heat...
- Fri Jan 20, 2017 11:34 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Piston Diagram [ENDORSED]
- Replies: 1
- Views: 501
Re: Piston Diagram [ENDORSED]
For that diagram, A refers to the area of the top of the cylinder (the circular part).
If you multiply that area by the distance the piston moves due to external pressure, you'd get the change in volume.
If you multiply that area by the distance the piston moves due to external pressure, you'd get the change in volume.
- Mon Jan 16, 2017 7:44 pm
- Forum: Phase Changes & Related Calculations
- Topic: Different enthalpy of vaporizations for water
- Replies: 3
- Views: 661
Re: Different enthalpy of vaporizations for water
The 40.7 degrees celsius value would be used when we're dealing with water at its boiling point (100 degrees celsius), as mentioned at the bottom of pg 283. The standard reaction enthalpy only indicates that the reactants and products are in their standard state (pure form at 1 bar/atm). Since tempe...

