Hi!
AgBr is essentially telling you that you have Ag+; you're just including the Br- with it (these two combine to be AgBr) because that was how it was given in the equation. So you could replace AgBr with Ag+. Hope this helps!
Search found 22 matches
- Sun Mar 19, 2017 8:25 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Midterm 2016 Q3C
- Replies: 2
- Views: 671
- Sun Mar 19, 2017 8:20 am
- Forum: *Ethers
- Topic: Exercise 2.43 (e) in Green course reader
- Replies: 1
- Views: 1347
Re: Exercise 2.43 (e) in Green course reader
Hi Sanjay!
If you count the carbons of the cyclohexane counterclockwise from the double bond, you should get the methoxy attached to the 4th carbon. :)
If you count the carbons of the cyclohexane counterclockwise from the double bond, you should get the methoxy attached to the 4th carbon. :)
- Sun Mar 12, 2017 8:12 pm
- Forum: *Electrophilic Addition
- Topic: Is something an electrophilic addition because of the first step or slowest step?
- Replies: 1
- Views: 1272
Re: Is something an electrophilic addition because of the first step or slowest step?
Hi Michael! It is an electrophilic addition reaction because step 1 involves the addition of an electrophile (which in this case is H^+) to the other molecule in the first step. For general electrophilic addition reactions, a pi (double) bond is broken and two sigma (single) bonds are formed. The bo...
- Sun Mar 05, 2017 3:16 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Homework 15.61
- Replies: 1
- Views: 523
Re: Homework 15.61
Hi! You would use the equation ln \frac{k_{2}}{k_{1}} =\frac{E_{a}}{R}*[\frac{1}{T}_{1}-\frac{1}{T_{2}}] , which can be derived by subtracting the Arrhenius equation for temperature 1 from the Arrhenius equation for temperature 2 (this eliminates A). For reference, see page 642 in the textbook! Hope...
- Sun Feb 26, 2017 10:53 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Textbook question 15.71
- Replies: 3
- Views: 1326
Re: Textbook question 15.71
OH- is a catalyst because it is already in the basic solution from the beginning of Step 1 (so it is not formed during the reaction). It still cancels out, meaning it is not consumed and can be used repeatedly.
- Sun Feb 19, 2017 10:38 pm
- Forum: General Rate Laws
- Topic: RXN rate graph question
- Replies: 1
- Views: 421
Re: RXN rate graph question
Hi Angela! I think this has to do with the difference between instantaneous rate of change and average rate of change. The line tangent to the curve is the instantaneous rate. It is more accurate because it's exactly one point on the curve, and delta t is practically 0. If you take the average of th...
- Sun Feb 12, 2017 7:57 pm
- Forum: Balancing Redox Reactions
- Topic: HW 14.1
- Replies: 1
- Views: 420
Re: HW 14.1
Hi Olivia! Pretty sure that's a typo in the book and it's supposed to be 14H+. In regards to how you get 8H+ in the end, you have to multiply part b) by 3 to get the same amount of electrons for both half-reactions before you can add them. This means you will have 6H+ on the products side, and 14H+ ...
- Sun Feb 05, 2017 11:34 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3635836
- Sun Jan 29, 2017 8:23 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: PRACTICE QUIZ 1 (#9)
- Replies: 2
- Views: 660
Re: PRACTICE QUIZ 1 (#9)
Hi!
My TA explained that entropy in the entire universe is always increasing, and ΔSsurr would have to take that into account.
My TA explained that entropy in the entire universe is always increasing, and ΔSsurr would have to take that into account.
- Sun Jan 22, 2017 9:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: #75
- Replies: 1
- Views: 522
Re: #75
Hello! I think your lewis structure for CH3CH(OH)CH3 is a bit off. The structure should have a total of 26 e-. The lewis structure that gives you a formation of a C=O bond will only have a total of 24 electrons, whereas if you attach the oxygen atom to only one carbon atom, you will get all 26 elect...
- Sun Jan 15, 2017 7:44 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3635836
- Sat Dec 03, 2016 1:32 am
- Forum: *Titrations & Titration Calculations
- Topic: Chapter 13 Question 25
- Replies: 1
- Views: 755
Re: Chapter 13 Question 25
Hi! Neutralization occurs at the stoichiometric point. For this titration, the stoichiometric point is where the moles of H^{+} of the added titrant (HCl) is equal to the moles of OH^{-} of the analyte (NaOH). Basically, we want to get the amount of moles of HCl = the amount of moles of NaOH. Just t...
- Sun Nov 27, 2016 4:15 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Drawing Structures for Final
- Replies: 1
- Views: 513
Re: Drawing Structures for Final
Hello! Since we didn't have to draw VESPR structures for the midterm, I don't think it will be on the final. I think all we need to know is how to identify the VSEPR formula, molecular shape, etc. However, drawing out the VESPR structures may help you identify the bond angles and the molecular shape...
- Sun Nov 20, 2016 7:56 pm
- Forum: Conjugate Acids & Bases
- Topic: Question 12.23
- Replies: 1
- Views: 499
Re: Question 12.23
Hi!
I think because the ratio of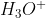 ions and
ions and 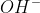 ions is 1:1 in the chemical equation:
ions is 1:1 in the chemical equation: \rightleftharpoons H_{3}O^{+}(aq)+ OH^{-}(aq)) and because the conditions for both ions are the exact same, they must have the same molarity.
and because the conditions for both ions are the exact same, they must have the same molarity.
I think because the ratio of
- Sun Nov 13, 2016 6:21 pm
- Forum: Ideal Gases
- Topic: Ideal Gas Law and Equilibrium Constant
- Replies: 7
- Views: 1602
Re: Ideal Gas Law and Equilibrium Constant
Increasing pressure (by decreasing the volume) will make your chemical equation favor the side with less gas particles. Fall 2015 Question 10 So in this problem, you only need to look at the gas molecules ( I_{2} is a solid). After you balance the chemical equation, you get: 2HCl (g) + I_{2}...
- Sat Nov 05, 2016 10:45 pm
- Forum: Hybridization
- Topic: Which Hybrid Orbital
- Replies: 6
- Views: 1138
Re: Which Hybrid Orbital
michaeljwilson3 wrote:if there is a double or triple bond, that only counts as one electron density correct?
Yes, double and triple bonds only count as one electron density!
For example,
- Sun Oct 30, 2016 12:11 am
- Forum: Hybridization
- Topic: Chapter 4 Question #81
- Replies: 1
- Views: 899
Re: Chapter 4 Question #81
Hey Karla! For the second part (hybridization of atoms), you want to count the regions of electron density of each atom, which will be equivalent to how many hybrid orbitals that atom has. In this case, both B and N have the same amount of hybrid orbitals. Each bond and lone pair of an atom counts a...
- Sat Oct 22, 2016 10:59 pm
- Forum: Hybridization
- Topic: Electronegativity, Electron Affinity and Ionization Energy
- Replies: 2
- Views: 24164
Re: Electronegativity, Electron Affinity and Ionization Energy
Hi Erik! To add on to what Eljie said: The trends of ionization energy, electron affinity, and electronegativity on the periodic table are the same. From left to right AND down to up on the table, the elements will have an increase in these trends. So if the ionization energy of an atom is high, the...
- Sun Oct 16, 2016 1:57 am
- Forum: Resonance Structures
- Topic: resonance structures ?
- Replies: 1
- Views: 639
Re: resonance structures ?
Hi Julianne, After a quick online search, I think it’s safe to say the only requirement needed to determine that an ion has resonance is that it has more than one Lewis Dot structure (that is, the ion/molecule cannot be expressed by only one structure). I found this web page that explains resonance ...
- Sun Oct 16, 2016 1:29 am
- Forum: Limiting Reactant Calculations
- Topic: Fundamentals M.9 [ENDORSED]
- Replies: 3
- Views: 913
Re: Fundamentals M.9 [ENDORSED]
Hi Parsia, Sorry for the late reply! In this question, you are given two compounds that react together to form two new compounds. This is called a double replacement/displacement reaction : AB + XY \rightarrow XB + AY The products are formed when the two cations (or two anions) of the reactants swit...
- Sun Oct 09, 2016 2:35 am
- Forum: Limiting Reactant Calculations
- Topic: Fundamentals M.9 [ENDORSED]
- Replies: 3
- Views: 913
Re: Fundamentals M.9 [ENDORSED]
Hi Michelle! What I do first is write out the complete equation: Cu(NO_{3})_{2} (aq) + 2 NaOH (aq) \rightarrow Cu(OH)_{2} (s) + 2 NaNO_{3} (aq) (We get 2 NaNO_{3} from the other products in the chemical reaction) Next, we want to write out the complete...
- Fri Sep 30, 2016 1:26 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Quiz 1, Preparation 2, Q1
- Replies: 1
- Views: 589
Re: Quiz 1, Preparation 2, Q1
Hi Ashley! To set up this problem you want to use the Molarity equation: \mathit{M} = \frac{\mathit{n}}{V} (Molarity = moles of solute/Liters of solution). We are given the Molarity ( 2 M NaOH) and the volume ( .3 L ) and we must solve for the moles of solute (NaOH). \mathit{2 M} = \frac{\mathit{n}}...

