Search found 33 matches
- Tue Mar 14, 2017 9:15 pm
- Forum: *Cycloalkenes
- Topic: Numbering
- Replies: 1
- Views: 1330
Re: Numbering
Hi! The rule is that you want the absolute lowest number on a substituent, not the lowest sum. As the course reader states 1<2, so the name including 1 is correct. Just for an example, if I as picking between a name that had the numbers 2 and 3 or a name that had the numbers 1 and 14 (not really sur...
- Tue Mar 14, 2017 10:50 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Final 2013 4B
- Replies: 1
- Views: 504
Re: Final 2013 4B
Hi! You are right in that this problem is definitely a tricky one. I think the logic of the solution is that we can find the reaction quotient and we can make an equation representing [O2] using the experiments. Step 1: Q= 1/[O2] comes from the overall reaction on the top of the left hand page. We d...
- Fri Mar 10, 2017 8:31 pm
- Forum: *Cycloalkanes
- Topic: Constitutional Isomers
- Replies: 2
- Views: 1065
Re: Constitutional Isomers
Constitutional isomers are compounds that have the same molecular formula and different connectivity. They will all have different names. So any cycloalkane where I can count up 8 carbons and 16 hydrogens is a constitutional isomer. One of the easiest ways to do this is to start with your basic cycl...
- Mon Mar 06, 2017 9:58 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: Final Winter 2014 #6 and 7
- Replies: 1
- Views: 513
Re: Final Winter 2014 #6 and 7
If you read the question carefully, you can see it says "Draw curved arrows in the mechanism below." This would imply that the mechanism is already drawn out and that one would have to draw the arrows in.
The rate law is given in the problem and does not need to be solved for.
The rate law is given in the problem and does not need to be solved for.
- Mon Mar 06, 2017 9:49 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Final Winter 2015 #6C
- Replies: 1
- Views: 456
Re: Final Winter 2015 #6C
Reaction coordinate is synonymous with reaction progress.
- Sat Mar 04, 2017 10:18 pm
- Forum: *Haloalkanes
- Topic: Introduction to Organic Chem ch. 4 #31
- Replies: 2
- Views: 1631
Re: Introduction to Organic Chem ch. 4 #31
Hi! In step one of the reaction two bonds are broken: pi bond in c=c double bond in propENE and H-Br sigma bond. In step two of the reaction not bonds are broken, rather one is formed. Since more bonds were broken in step one than step two, the activation energy of step 1 will be greater than step 2...
- Thu Mar 02, 2017 8:39 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalyst
- Replies: 16
- Views: 2434
Re: Catalyst
If the catalyst is in the rate determining step, then it must be listed in the overall rate law because its presence, or lack of, will affect the rate of reaction.
- Sun Feb 26, 2017 7:00 pm
- Forum: *Electrophilic Addition
- Topic: Cholesterol example on page 84 of CR
- Replies: 2
- Views: 643
Re: Cholesterol example on page 84 of CR
The hydrogens are omitted because the line diagram would be too complex with them. They are shown in some places because those are the regions we are inspecting.
- Sun Feb 26, 2017 6:47 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Determining k vs k'
- Replies: 3
- Views: 777
Re: Determining k vs k'
Typically k is the rate constant for the forward reaction and k' is the rate constant for the reverse reaction. 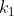 can also represent the forward reaction rate constant and
can also represent the forward reaction rate constant and 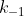 represents that of the reverse. In practice though, as long as you define what your rate constant is you're okay.
represents that of the reverse. In practice though, as long as you define what your rate constant is you're okay.
- Tue Feb 21, 2017 8:48 pm
- Forum: Second Order Reactions
- Topic: Quiz 2 Practice Question 7
- Replies: 3
- Views: 822
Re: Quiz 2 Practice Question 7
Hi! The differential rate law is not fit for this sort of problem because the variable of time is not included. -\frac{d[A]}{dt}=k[A]^{n} Which says that the concentration of A decreases over time in proportion to the concentration of A (itself), raised to the power of n. Where n is called the order...
- Tue Feb 21, 2017 8:19 pm
- Forum: First Order Reactions
- Topic: 15.25
- Replies: 1
- Views: 520
Re: 15.25
Hi! I don't think there is any particular advantage either way. On one hand by dividing by negative k you need to remember to flip A/A0 to A0/A. But one the other, if you divide by positive k you need to remember that your answer will come out as a negative time and that it needs to be positive in y...
- Tue Feb 14, 2017 7:16 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: midterm 2015 #4&5 B
- Replies: 3
- Views: 869
Re: midterm 2015 #4&5 B
If you look closely you can see that the problem is divided into two parts. The entropy change due to temperature ALONE is calculated. We make volume constant in this case. Then the entropy change due to volume ALONE is calculated. Since we are only considering a change in volume we can use equation...
- Mon Feb 13, 2017 6:17 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Molar Entropy
- Replies: 4
- Views: 1308
Re: Molar Entropy
Hi! I have looked into your question as I was wondering the same thing as well. For starters, in the quiz it mentions that T=0K so we know that nothing is moving/vibrating. When T=0K I believe (someone correct me if I'm wrong) that we are only to look at the structure because nothing is moving. A wa...
- Sun Feb 12, 2017 11:04 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Winter 2013 Midterm Q7
- Replies: 2
- Views: 597
Winter 2013 Midterm Q7
Hi! The question asks to calculate K and dG0 at 298 K for the following redox reaction: Mn2+ + Br2 --> MnO4- +Br- I was able to solve for dG0 by using dG0 = -nFE0 and obtained the same answer as the answer key. When I try to solve for K using dG0 = -RTln(K) I find the K is equal to 1.35x10^-72. The ...
- Sat Feb 11, 2017 4:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Book Question 8.55
- Replies: 1
- Views: 381
Re: Book Question 8.55
Hi!
The equations are already balanced. On the reactants side there are 2 bariums and 2 oxygens. On the products side there are 2 bariums and 2 oxygens. No further manipulation is needed.
The equations are already balanced. On the reactants side there are 2 bariums and 2 oxygens. On the products side there are 2 bariums and 2 oxygens. No further manipulation is needed.
- Mon Jan 30, 2017 3:21 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Why isn't q zero during isothermal reactions?
- Replies: 3
- Views: 4825
Re: Why isn't q zero during isothermal reactions?
Hi! Let's not forget the \Delta U=q+w ! As you stated, for isothermal processes dU is zero. We can rearrange the formula to look like this: q=-w So if we said q was zero, this wouldn't make sense. It would be as if nothing is happening because your change in internal energy is zero, your work is zer...
- Sun Jan 29, 2017 7:34 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Quiz 1 Prep # 9
- Replies: 2
- Views: 1045
Re: Quiz 1 Prep # 9
Hi! I am having difficulty understanding why dS(total) is positive. What are the cues in the question that give this away? Furthermore, if a reaction is spontaneous (dS(total) >0) why is it that dG is not less than zero? I think I'm a little mixed up...if anyone could help answer my questions I woul...
- Mon Jan 23, 2017 8:44 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Homework Question 15
- Replies: 1
- Views: 9749
Re: Homework Question 15
Hi! First thing to remember is that \Delta S^{\circ}=\frac{\Delta H^{\circ}}{T} , where in this case deltaH will be the enthalpy of fusion. The enthalpy of fusion of ice is 6.01 kJ.mol-1 and in this case since we are freezing water it will be -6.01 kJ.mol-1. The value on your periodic table is going...
- Thu Jan 19, 2017 10:35 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Help With Question 8.39 [ENDORSED]
- Replies: 2
- Views: 605
Re: Help With Question 8.39 [ENDORSED]
Hello! The value of 6.01 kJ/mol is the enthalpy of fusion for water. I would not concern yourself with Gibbs Free Energy at the moment. You need to use the enthalpy of fusion because your starting sample is ICE and you want your final to be a LIQUID. In other words, the enthalpy of fusion is for whe...
- Sun Jan 15, 2017 10:47 pm
- Forum: Phase Changes & Related Calculations
- Topic: HW 8.67 [ENDORSED]
- Replies: 5
- Views: 1047
Re: HW 8.67 [ENDORSED]
Sorry about the confusion, let me state the question in its entirety. The question states: "Use the information in Tables 8.3, 8.6, and 8.7 to estimate the enthalpy of formation of each of the following compounds in the liquid state." I am specifically interested in how to do this for H2O....
- Sun Jan 15, 2017 6:33 pm
- Forum: Phase Changes & Related Calculations
- Topic: HW 8.67 [ENDORSED]
- Replies: 5
- Views: 1047
HW 8.67 [ENDORSED]
Hi all. I am stuck on letter a) of number 67 of the chapter 8 homework. Looking at the solution manual, I understand the equation and all the calculations that lead to the change in enthalpy being -242kJ/mol. However, I think I am misunderstanding what this value represents. Why is it that -242kJ/mo...
- Fri Jan 13, 2017 9:09 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3611068
Re: Post All Chemistry Jokes Here
What kind of fish is made of sodium?
2Na!
2Na!
- Mon Nov 28, 2016 11:55 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Homework Help 12.33
- Replies: 1
- Views: 676
Re: Homework Help 12.33
Hi! Part A: To answer your question about where the pH of 13.25 came from, it was given in the problem. Since we know that 14 = pH + pOH and that the pH is 13.25, we can find that the pOH is 0.75. Given the pOH we can find the concentration of hydroxide ions by remembering some log rules. We know th...
- Wed Nov 23, 2016 11:47 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3611068
Re: Post All Chemistry Jokes Here
Q: What is the name of 007's Eskimo cousin?
A: Polar Bond
A: Polar Bond
- Tue Nov 15, 2016 10:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: calculating Kc [ENDORSED]
- Replies: 3
- Views: 715
Re: calculating Kc [ENDORSED]
Hi! I am sure you know how to solve this problem because it's just an ICE box problem is disguise. I am going to put a disclaimer at the beginning that I cannot figure what sig fig rules the solution uses so my answer will be slightly off. If someone could clarify what rounding needs to occur to get...
- Thu Nov 10, 2016 10:48 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: NH2 Bonds
- Replies: 2
- Views: 2803
Re: NH2 Bonds
Hi! The above response is correct, however I would like to mention something that may clear up the situation further. I believe that Professor Lavelle mentioned that there is one pair of electrons on NO2 that contributes to bonding in a transition metal complex. While there are two lone pairs, ammin...
- Fri Nov 04, 2016 4:22 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3611068
Re: Post All Chemistry Jokes Here
A photon walks into a hotel. The desk clerk says, "Welcome to our hotel. Can we help you with your luggage?" The photon says, "No thanks, I'm traveling light."
Happy Friday!
Happy Friday!
- Mon Oct 24, 2016 9:43 pm
- Forum: Electronegativity
- Topic: Electron Affinity
- Replies: 12
- Views: 2119
Re: Electron Affinity
Hi! Electron affinity is how much an atom wants to gain an electron. An atom will want to gain an electron if is super close to obtaining a half full orbital, a full orbital, or an octet in terms of valence electrons. In terms of periodicity, electron affinity increase from left to right and bottom ...
- Mon Oct 17, 2016 10:52 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Chapter 2 Question 29 [ENDORSED]
- Replies: 3
- Views: 778
Re: Chapter 2 Question 29 [ENDORSED]
Hi! I know someone has already kindly answered your question, but I thought you might like another explanation. (: A. Though the problem gives you n, you only really need l to find the number of electrons in this case. Ml is all the integral values of l, in this case -1, 0, 1. In each of these subor...
- Mon Oct 17, 2016 11:01 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 2.1
- Replies: 6
- Views: 3543
Re: 2.1
Hi! You are correct in stating that when an orbital goes from (n)S --> (n+1)P the value of n, l and the radius increase. You can tell the value of n increases because you can look at the "coefficient" of the letter. In this case it increased from 1 to 2. Since the value of l is calculated ...
- Mon Oct 10, 2016 8:51 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Post-Module, Question 17 [ENDORSED]
- Replies: 2
- Views: 767
Re: Atomic Spectra Post-Module, Question 17 [ENDORSED]
Think of the question asking you to find the length of each individual "part" that makes up one meter, given the total number of "parts." You would do this by dividing (partitioning) one meter by the total number of parts given. Having said this, replace the word part with "...
- Mon Oct 03, 2016 10:59 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Video Module #18
- Replies: 1
- Views: 415
Re: Atomic Spectra Video Module #18
Hi! I can see why you are confused when you look at this problem as there is an excess of information given. To calculate the energy per photon all we need is Plank's constant, the speed of light and the wavelength (which is given as 1850 nm). E = \frac{hc}{\lambda } E = \frac{6.62608x10^{-34}Js*2.9...
- Mon Sep 26, 2016 10:42 pm
- Forum: Properties of Light
- Topic: Wavelength and Energy
- Replies: 3
- Views: 1873
Re: Wavelength and Energy
Hi! Question 23 is asking you to calculate the wave length of these gamma waves given the energy in KeV (Kiloelectronvolts). The first step is to convert from KeV to Joules, the SI unit for energy. 140.511 KeV * \frac{1.6022x10^{-19} J}{1 KeV} = 2.513x10^{-14} J Now we have the energy of the gamma r...

