Can be volume change in a closed system?
I read that there can be, for example if there's a piston but I'm not sure. But if that's true then why on #2 in the midterm is U=0 in a closed system if the volume can change? Even though q=0, can't there be work?
Search found 9 matches
- Sun Mar 19, 2017 12:42 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed system and Midterm
- Replies: 3
- Views: 892
- Mon Mar 13, 2017 3:54 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Details and Review Sessions Winter 2017
- Replies: 114
- Views: 27204
Re: Final Exam Details and Review Sessions Winter 2017
Do we need to know how to draw and name functional groups attached to cyclic carbon structures like carbaldehyde?
- Sun Mar 05, 2017 3:14 pm
- Forum: *Alkanes
- Topic: Naming [ENDORSED]
- Replies: 93
- Views: 16843
Re: Naming [ENDORSED]
There was also a thing Lavelle mentioned about not using a dash sometimes for the second to last term? It's in our course reader but was different on the powerpoint or something like that. Yeah the last carbon group doesn't need a dash, it just combines with the parent group, for example 3-ethyl-2,...
- Wed Feb 22, 2017 11:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Quiz 2 Winter 2017
- Replies: 160
- Views: 25033
Re: Quiz 2 Winter 2017. Help on Quiz 2 Prep please!
It is important to distinguish between the reaction and the rate constant. The units of rate constant are always M * s^-1. True or False. I think the answer is True, however I'm not sure about this question. Could someone please explain? I think the rate units are always M*s^-1, but the units of ra...
- Thu Feb 09, 2017 10:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.13 part d
- Replies: 1
- Views: 474
14.13 part d
How do we write the half reactions for \rightarrow Au(s)+Au^{3+}(aq)) and the cell diagram for this? I know the solutions manual gives the answer but I'm still confused as to how to do it
and the cell diagram for this? I know the solutions manual gives the answer but I'm still confused as to how to do it
- Sun Feb 05, 2017 1:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 2
- Views: 444
Re: Cell Diagram
Hi, I think the textbook mentions that the reactant should be on the left and products on the right. For anode/cathode, if electrons are flowing to the right (+ cell potential), then the anode is on the left and cathode on the right. If electrons are flowing to the left (- cell potential), then the ...
- Sat Jan 21, 2017 12:43 pm
- Forum: Calculating Work of Expansion
- Topic: entropy equations
- Replies: 3
- Views: 574
Re: entropy equations
But I don't think W is work? It's the W for degeneracy I believe
- Fri Jan 20, 2017 11:27 pm
- Forum: Calculating Work of Expansion
- Topic: entropy equations
- Replies: 3
- Views: 574
entropy equations
In class we talked about how 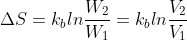
does that mean W = V, and why is that?
does that mean W = V, and why is that?
- Sun Jan 15, 2017 10:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpy versus standard Enthalpies of formation
- Replies: 2
- Views: 549
Bond Enthalpy versus standard Enthalpies of formation
How do we distinguish between when to use bond Enthalpy or standard Enthalpy?

