Search found 16 matches
- Thu Mar 16, 2017 8:47 pm
- Forum: *Alkenes
- Topic: Naming Convention for Double Bonds
- Replies: 1
- Views: 1331
Naming Convention for Double Bonds
When we name an organic molecule with a double bond, do we always have to identify where the double bond is located?
- Wed Mar 08, 2017 10:47 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616885
- Tue Feb 14, 2017 3:41 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Winter 2013 Midterm #7: Which Nernst Equation to Use?
- Replies: 2
- Views: 646
Winter 2013 Midterm #7: Which Nernst Equation to Use?
#7 of the Winter 2013 practice midterm uses the Nernst equation E = E˚ - \frac{0.05916V}{n} logQ to solve for K. Why isn't the other equation E = E˚ - \frac{RT}{nF} lnQ used? When I used the second equation, the answer wasn't the same as when I used the first. How do you know when to use which equat...
- Sat Feb 11, 2017 5:25 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Quiz One - #5 Calculating Heat
- Replies: 2
- Views: 585
Re: Quiz One - #5 Calculating Heat
I think we have the same quiz--melting a -55˚C block of ice into 25˚C water? When you first melt ice from -55˚to 0˚, you use the specific heat capacity of ice in the equation q = mc∆T. During the phase change from ice to water (at 0˚), you use the heat of fusion in the equation q = n∆H fus (note tha...
- Thu Jan 26, 2017 11:03 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Quiz 1 Prep Question 3
- Replies: 4
- Views: 966
Re: Quiz 1 Prep Question 3
Since both pressure and volume are changing (implying constant temp), you would use the equation W = -nRT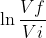 . For all reversible reactions, ∆U = 0. You can use these two equations to solve for w, q, and ∆U.
. For all reversible reactions, ∆U = 0. You can use these two equations to solve for w, q, and ∆U.
- Wed Jan 25, 2017 12:12 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Molar Heat Capacity for Phase Change of Water
- Replies: 1
- Views: 492
Molar Heat Capacity for Phase Change of Water
At constant pressure, why is Cp (s) > Cp (l) > Cp (g) for the phase change of water?
- Mon Jan 16, 2017 12:09 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3616885
Re: Post All Chemistry Jokes Here
Organic chemistry is difficult. Those who study it have alkynes of trouble.
- Sat Dec 03, 2016 7:49 pm
- Forum: *Titrations & Titration Calculations
- Topic: Weak Acid Titration Equilibrium Table Equation? [ENDORSED]
- Replies: 1
- Views: 783
Weak Acid Titration Equilibrium Table Equation? [ENDORSED]
When you're trying to find the pH at the stoichiometric point of a titration of a weak acid and a strong base, why do you use the reaction between the salt and H 2 O as the chemical equation for the equilibrium table? Why don't you use the reaction between the weak acid and strong base? For example,...
- Tue Nov 15, 2016 12:42 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Fall 2014 Quiz Prep #8
- Replies: 1
- Views: 456
Re: Fall 2014 Quiz Prep #8
You don't need to use PV=nRT for this problem! :) Just use the ICE table Since you already set the equilibrium constant expression, I'm assuming you got: K p = P NH3 (P H2S ) = 0.11 Using the ICE table, you would ignore NH 4 HS (because it's a solid) and just focus on NH 3 & H 2 S Initial partia...
- Sat Nov 12, 2016 2:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Clarification on R and P Calculations
- Replies: 2
- Views: 540
Re: Clarification on R and P Calculations
The brackets indicate the molar concentrations of the R and P, not molar masses.
Kp measures equilibrium for gas pressures, so you use the partial pressures of R and P.
Solids and liquids aren't included in the equilibrium calculations because their molar concentrations don't change in a reaction.
Kp measures equilibrium for gas pressures, so you use the partial pressures of R and P.
Solids and liquids aren't included in the equilibrium calculations because their molar concentrations don't change in a reaction.
- Sat Nov 12, 2016 1:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: What is a Ligand?
- Replies: 3
- Views: 1470
What is a Ligand?
I'm not sure what exactly a ligand is. Is it just any atom attached to a central atom of a molecule? Or is a ligand specifically a transition metal, polyatomic ion, etc?
Thank you!
Thank you!
- Tue Oct 25, 2016 12:22 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Workbook: Quiz 2 Prep #4
- Replies: 3
- Views: 771
Workbook: Quiz 2 Prep #4
For the electron configuration of Cl - using noble gas abbreviation, should it be [Ne] 3s 2 3p 6 or simply [Ar]? My high school AP chem teacher taught me the first is the correct configuration and the second is not quite correct. Should the configuration be using [Ne] or are both correct? Thank you!
- Fri Oct 21, 2016 4:09 pm
- Forum: Lewis Structures
- Topic: Lewis Structure regarding the angles
- Replies: 2
- Views: 756
Re: Lewis Structure regarding the angles
In class, Dr. Lavelle used the example of BF 3 for a trigonal planar structure. For your case, let's say one of the F atoms is Cl. Since Cl is a bigger atom than F (thus more repulsion), it would cause the angle between the Cl and F atoms to become bigger. Knowing that the angle between atoms of a t...
- Tue Oct 11, 2016 3:27 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Question about Uncertainty in Velocity [ENDORSED]
- Replies: 1
- Views: 573
Question about Uncertainty in Velocity [ENDORSED]
When a problem says that ∆v is ± ____ m/s, why do you multiply the ∆v value by 2 when you're solving the uncertainty equation?
- Mon Oct 10, 2016 4:09 pm
- Forum: Photoelectric Effect
- Topic: Ch. 1 HW #33c
- Replies: 1
- Views: 532
Re: Ch. 1 HW #33c
The answer you found for part b, E = 1.66 x 10 -17 J, is the work function (threshold energy). In part c, you're trying to find the energy of the photon, which is: E photon = work function (threshold energy) + E k Plug in the answer from part b in to the the work function, and plug in the mass and v...
- Sat Oct 01, 2016 11:23 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Clarification for G.7
- Replies: 2
- Views: 705
Clarification for G.7
You need to prepare 510. g of an aqueous solution containing 5.45% KNO3 by mass. Describe how you would prepare the solution and what mass of each component you would use. Is the question saying that there is 510. g of substance that is dissolved into solution? Or is 510. g of solution is measured o...